Polymer drug carrier with redox response and AIE effect, drug-loaded micelle and preparation method thereof
A technology for polymers and drugs, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of slow drug release, limited application, lack of intelligent release, etc., and achieve cycle time. The effect of improving and improving biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A polymer drug carrier with redox response and aggregation-induced luminescent effect, the preparation method of which comprises the following steps:
[0047] (1) Preparation of polyamino acid benzyl ester macromolecular initiator
[0048] Weigh 5g of L-glutamic acid-γ-benzyl ester-N-carboxylic acid anhydride monomer, dissolve it in 50mL of anhydrous N,N-dimethylformamide, then add 0.209g of N-(2-aminoethyl) -2-Bromoisobutyramide was used as an initiator, and under the protection of nitrogen, the reaction was stirred at room temperature for 72 hours. After the reaction was completed, the reaction product was concentrated by distillation under reduced pressure, and then precipitated in ether for 3 times, and finally dried in vacuum to obtain formula II. macromolecular initiator;
[0049]
[0050] Wherein, the degree of polymerization n of the polyamino acid block is 18;
[0051] (2) Preparation of two-block polymer precursors
[0052] Take the resultant of step (1)...
Embodiment 2
[0064] The method for preparing polymer drug-loaded micelles by using the polymer drug carrier with redox response and AIE effect prepared in Example 1 comprises the following steps:
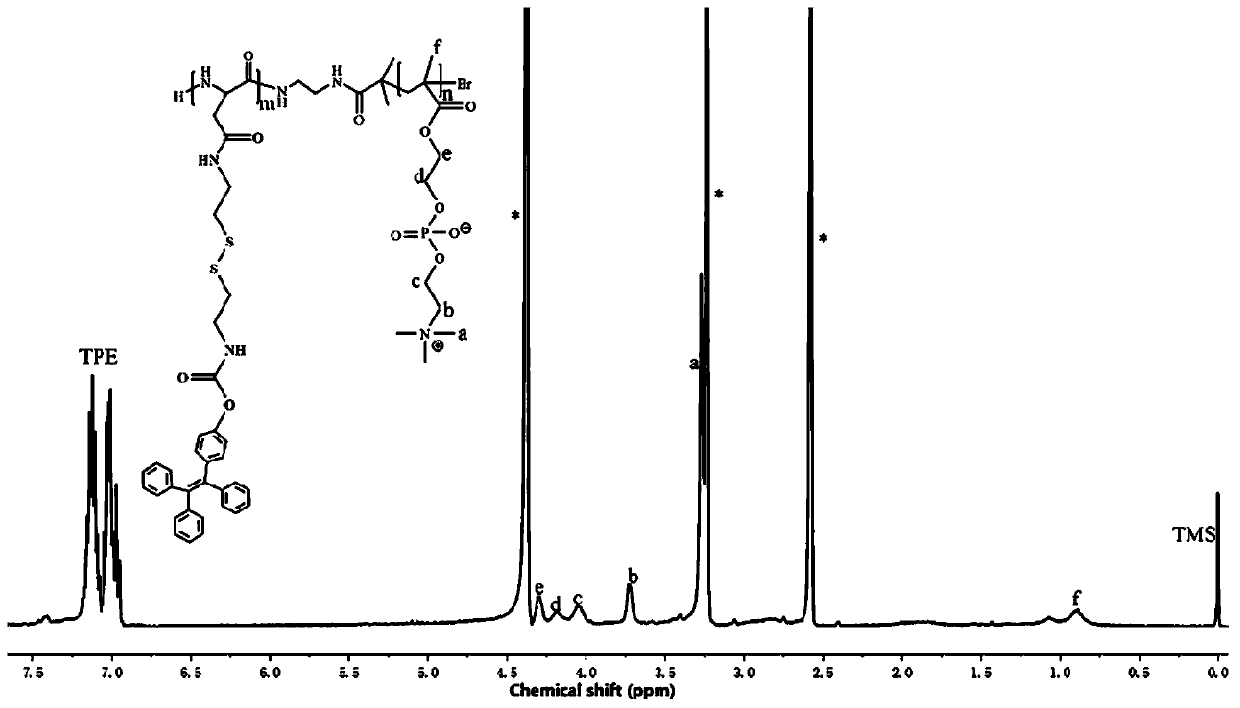
[0065] Weigh 10 mg of the obtained product of Example 1, dissolve it in the mixed solution of N,N-dimethylformamide and methanol in a volume ratio of 1:1, then add 2 mg of doxorubicin hydrochloride and 50 μL of triethylamine, Stir at room temperature for 12 hours, slowly add it dropwise into rapidly stirred ultrapure water, stir for 6 hours, and then dialyze to remove the organic solvent to obtain polymer drug-loaded nanomicelles. The specific structural formula is shown in Formula I:
[0066]
[0067] Wherein, the R* group is doxorubicin.
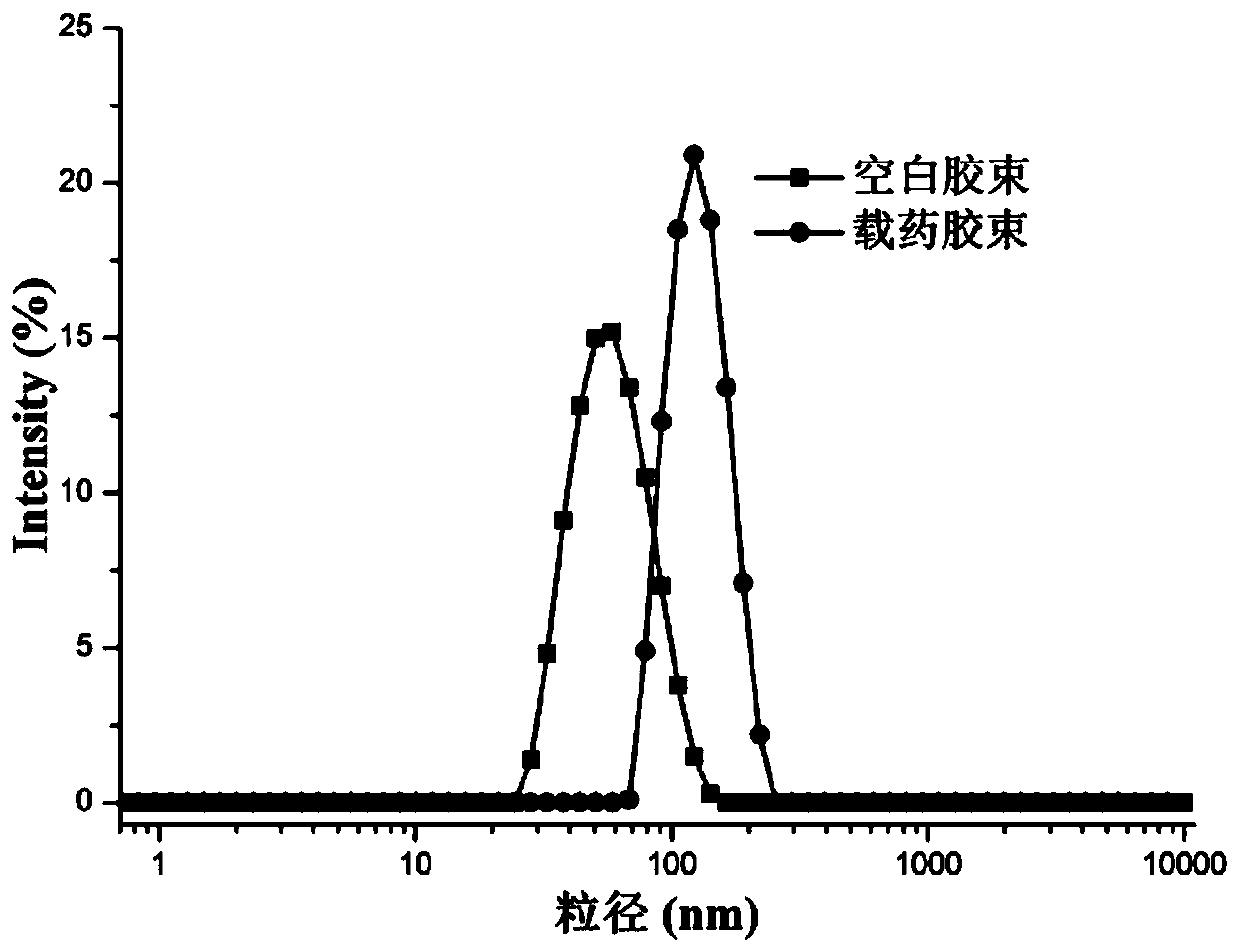
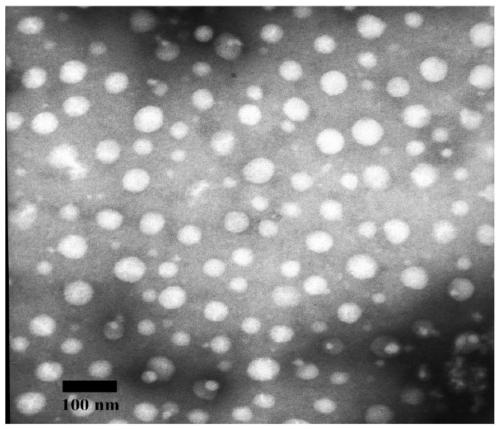
[0068] Dynamic light scattering instrument (DLS) measures the particle size of blank polymer micelles and polymer drug-loaded nano micelles, and the results are shown in figure 2 , the transmission electron microscope (TEM) image is shown in image 3 ....
Embodiment 3
[0071] A polymer drug carrier with redox response and aggregation-induced luminescent effect, the preparation method of which comprises the following steps:
[0072] (1) Preparation of polyamino acid benzyl ester macromolecular initiator
[0073] Weigh 20mmol L-glutamic acid-γ-benzyl ester-N-carboxylic acid anhydride monomer, dissolve it in 70mL anhydrous N,N-dimethylformamide, then add 0.1mmol N-(2-aminoethyl) -2-Bromoisobutyramide was used as an initiator, and under the protection of nitrogen, the reaction was stirred at room temperature for 72 hours. After the reaction was completed, the reaction product was concentrated by distillation under reduced pressure, and then precipitated in ether for 3 times, and finally dried in vacuum to obtain formula II. macromolecular initiator;
[0074]
[0075] Wherein, the degree of polymerization of the polyamino acid benzyl ester block is 15;
[0076] (2) Preparation of two-block polymer precursors
[0077] Take 0.5 g of the resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com