Method for synthesizing 3,4-dinitropyrazole by using micro-channel reactor
A technology of microchannel reactor and dinitropyrazole, which is applied in chemical instruments and methods, chemistry/physics/physicochemical reactors, organic chemistry, etc., can solve the problems of reduced purity of nitropyrazole, unstable product properties, It is unfavorable for continuous production and other problems, and achieves the effect of reducing production risk, reducing production and by-product generation, and avoiding separation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
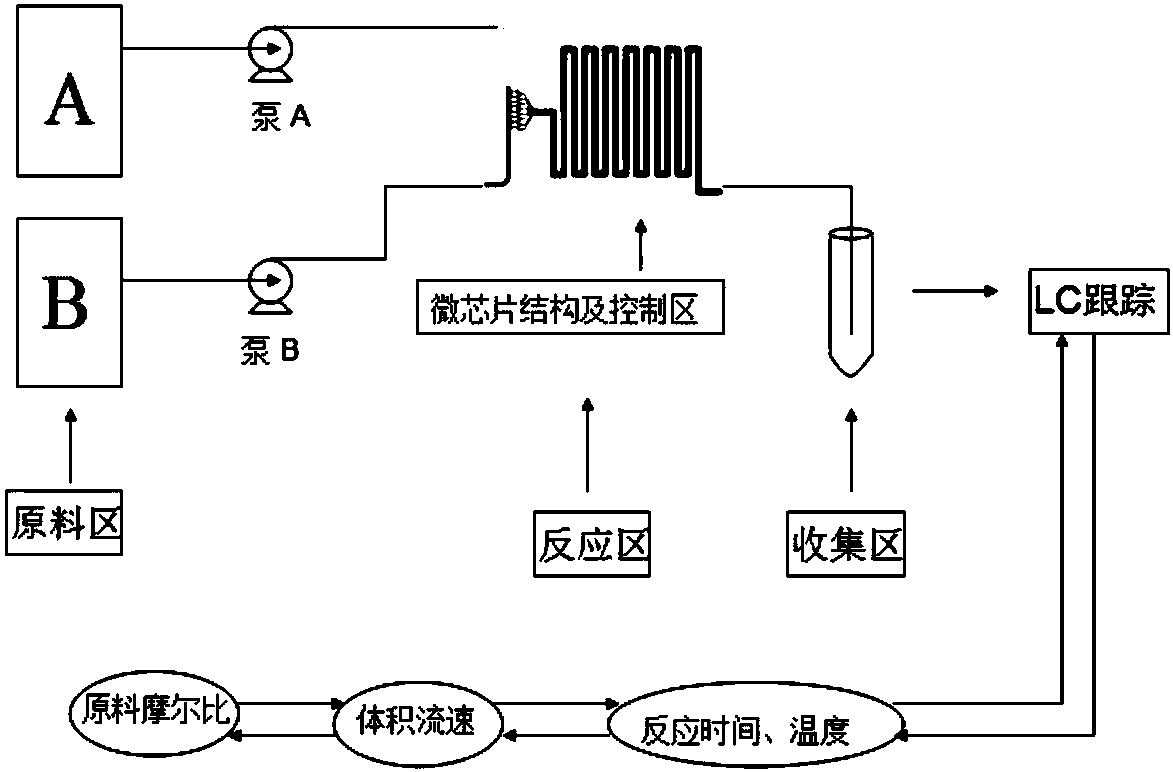
Image
Examples
Embodiment 1
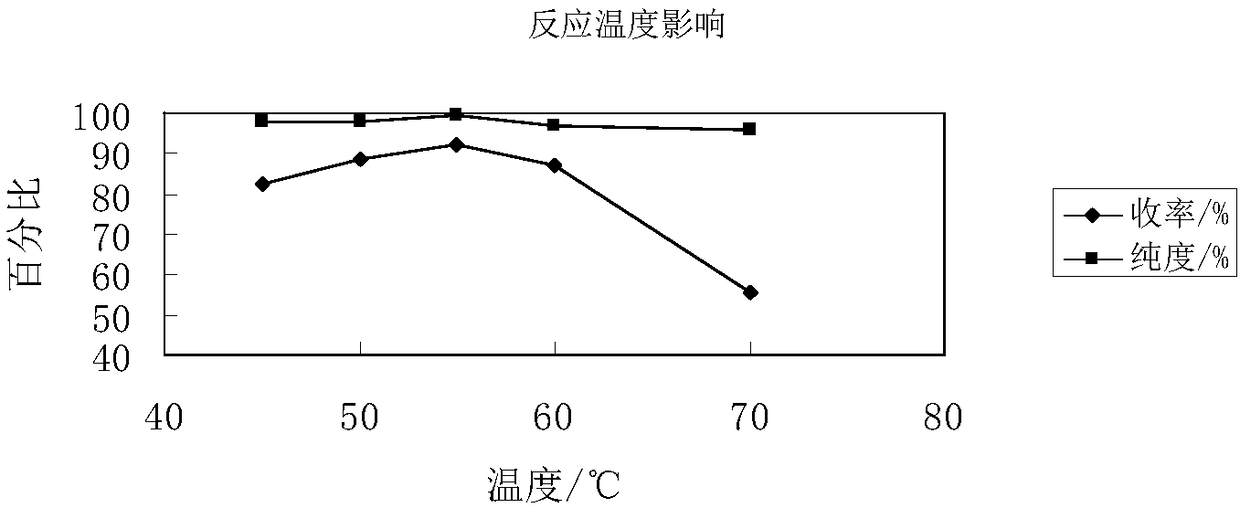
[0036] Slowly mix acetic anhydride and fuming nitric acid in an ice-water bath at a volume ratio of 5.5:1 to obtain a nitrating agent, and transport the acetic acid solution of the nitrating agent and pyrazole to the microchannel reactor via a high-pressure constant-flow pump with precise flow control. Two inlets, the molar ratio of nitric acid and pyrazole is controlled to be 1.1:1, and the two liquids are mixed and contacted in the microchannel reactor at 45-70°C for a moment and react, and the reaction solution is poured into crushed ice and filtered. Wash with ice water and dry in vacuum to obtain N-nitropyrazole; the flow rate of the acetic acid solution of pyrazole is 0.1 mL / min. N-nitropyrazole yield, purity are shown in Table 1 and figure 2 .
[0037] Table 1
[0038]
[0039] When the reaction temperature is lower than 45°C, there will be solids in the microreactor that will cause pipe blockage; as the reaction temperature increases, the yield and purity of N-ni...
Embodiment 2
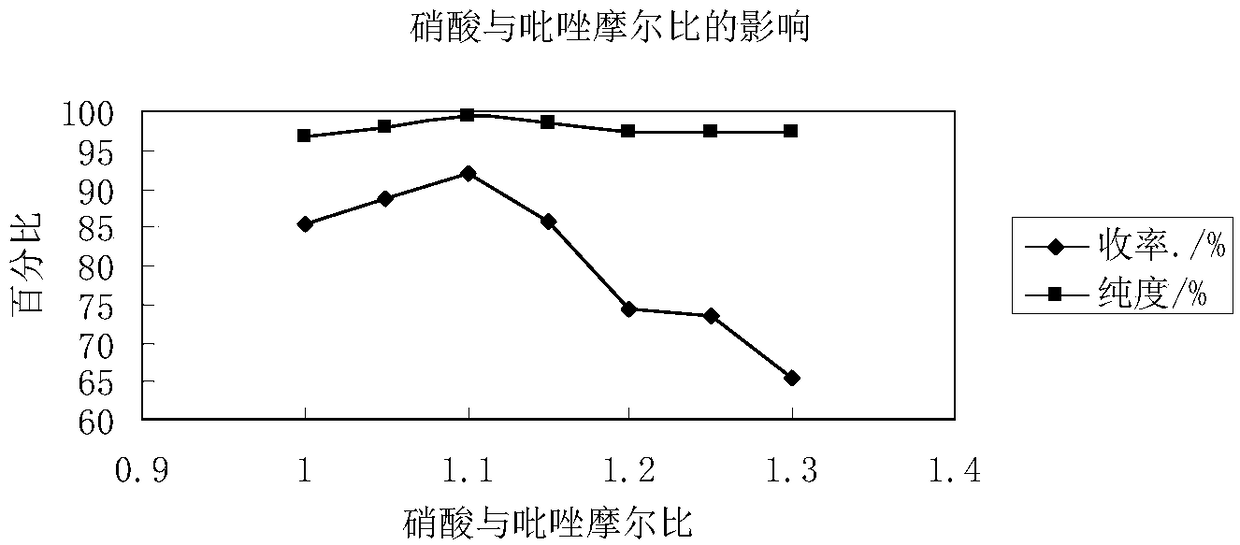
[0041] Slowly mix acetic anhydride and fuming nitric acid in an ice-water bath at a volume ratio of 5.5:1 to obtain a nitrating agent, and transport the acetic acid solution of the nitrating agent and pyrazole to the microchannel reactor via a high-pressure constant-flow pump with precise flow control. Two inlets, the molar ratio of nitric acid and pyrazole is controlled to be 1-1.3:1, and the two liquids are mixed and contacted in the microchannel reactor at 55°C for a moment and react, and the reaction solution is poured into crushed ice and filtered. Wash with ice water and dry in vacuum to obtain N-nitropyrazole; the flow rate of the acetic acid solution of pyrazole is 0.1 mL / min. N-nitropyrazole yield, purity are shown in Table 2 and image 3 .
[0042] Table 2
[0043]
[0044] When the amount of nitric acid was less, along with the increase of nitric acid and pyrazole mol ratio, more nitric acid participated in the reaction, and the yield and purity of N-nitropyrazol...
Embodiment 3
[0046] Slowly mix acetic anhydride and fuming nitric acid in an ice-water bath at a volume ratio of 3 to 6:1 to obtain a nitrating agent, and transport the nitrating agent and the acetic acid solution of pyrazole to the microchannel reaction via a high-pressure constant-flow pump with precise flow control The two inlets of the reactor, the molar ratio of nitric acid and pyrazole is controlled to be 1.1:1, and the two liquids are mixed and contacted in the microchannel reactor at 55 °C for a moment and react, and the reaction solution is poured into crushed ice and filtered. Wash with ice water and dry in vacuum to obtain N-nitropyrazole; the flow rate of the acetic acid solution of pyrazole is 0.1 mL / min. N-nitropyrazole yield, purity are shown in Table 3 and Figure 4 .
[0047] table 3
[0048]
[0049] Along with acetic anhydride and nitric acid volume ratio constantly increase, the yield and the purity of N-nitropyrazole also increase gradually; When nitric acid and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com