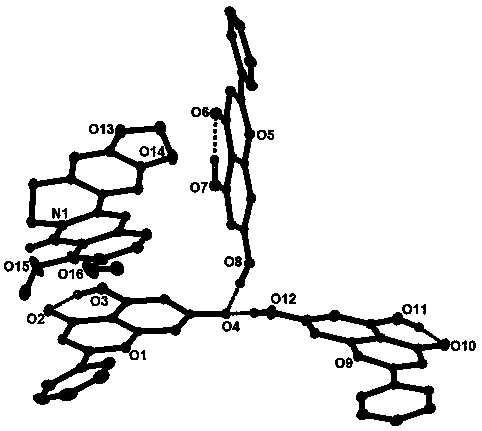
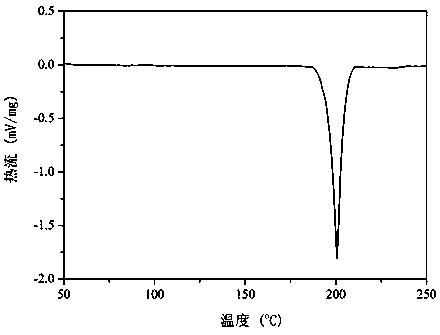
Berberine-chrysin pharmaceutical eutectic crystal and preparation method thereof
A technology of chrysin and berberine, applied in the field of berberine-chrysin drug co-crystal and its preparation, achieving the effect of simple and easy preparation method and clear crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 0.371 g of berberine hydrochloride, 0.254 g of chrysin and 0.04 g of sodium hydroxide in 20 mL of absolute ethanol respectively, mix and stir for 1 hour, add 0.254 g of chrysin in batches, and continue stirring for 2 hours to obtain a large amount of yellow precipitate , suction filtration, the obtained precipitate was washed with absolute ethanol, and dried in the air; the obtained powder was recrystallized in absolute ethanol to obtain a berberine-chrysin drug cocrystal.
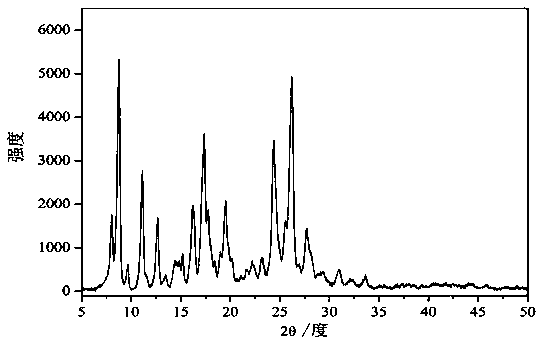
[0028] figure 1 It is the XRD figure of the berberine-chrysin drug cocrystal crystal prepared in the present embodiment. Depend on figure 1 It can be seen that the prepared crystals, with a diffraction angle of 2 θ ° ± 0.1 is expressed as: 8.2°, 8.9°, 9.8°, 11.3°, 11.8,° 12.8°,13.7°, 14.5°, 14.8°, 15.5°, 16.3°, 16.5°, 17.3°, 17.6°, 18.0° , 18.4°, 18.7°, 19.3°, 19.5°, 19.8°, 20.1°, 20.5°, 21.5°, 22.0°, 22.4°, 22.9°, 23.4°, 23.6°, 24.3°, 24.7°, 25.0°, 26.0 °, 26.6 °, 27.4 °, 28.1 °, 28...
Embodiment 2
[0033] Pharmacokinetic parameters of berberine-chrysin co-crystal in rats:
[0034] SD male rats with a body weight of 190-210 g were reared under conventional feeding conditions, free to drink water, and after fasting for 12 hours, press
[0035] 24 mg / kg (chrysin dosage) was administered orally, and blood was collected from the retroocular venous plexus at 0.10, 0.25, 0.25, 0.75, 1, 2, 3, 6, 8, 10, and 12 h before and after administration About 0.5 ml, centrifuge at 4000 rpm for 10 min. Take 200 μl of plasma, add 400 μl of methanol, vortex for 2 minutes, centrifuge at 10,000 rpm for 10 minutes, take the supernatant, and blow dry with nitrogen. Add 100 μl of mobile phase (methanol:water=50:50), vortex for 1 min, centrifuge at 10,000 rpm for 1 min, and take 40 μl of the supernatant for HPLC-MS detection. The HPLC-MS detection system is Aligent 1260 LC-6410 MS high-performance liquid chromatography system, and the chromatographic column is Ultimate XB-C 18 (2.1×50 mm, 3.5 μm),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com