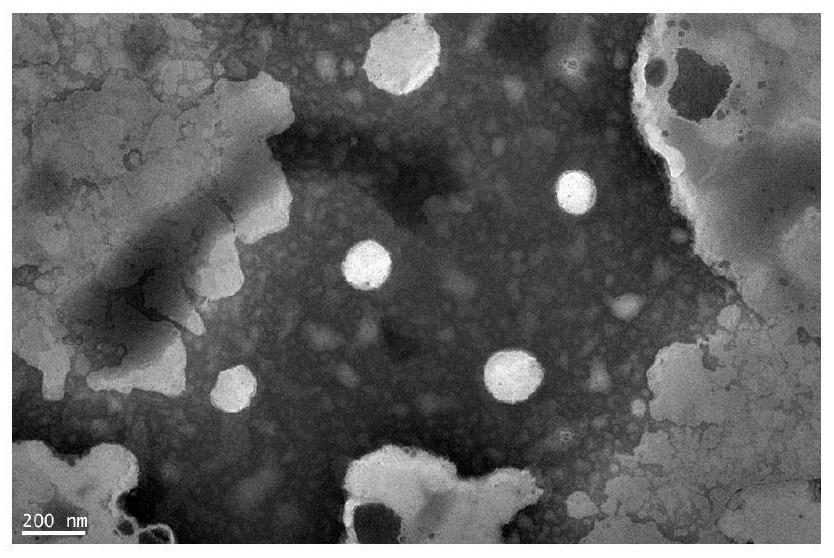
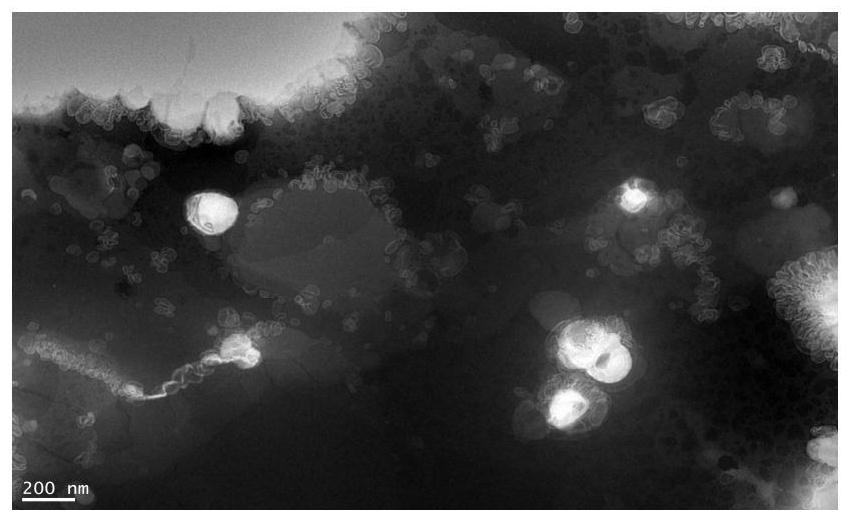
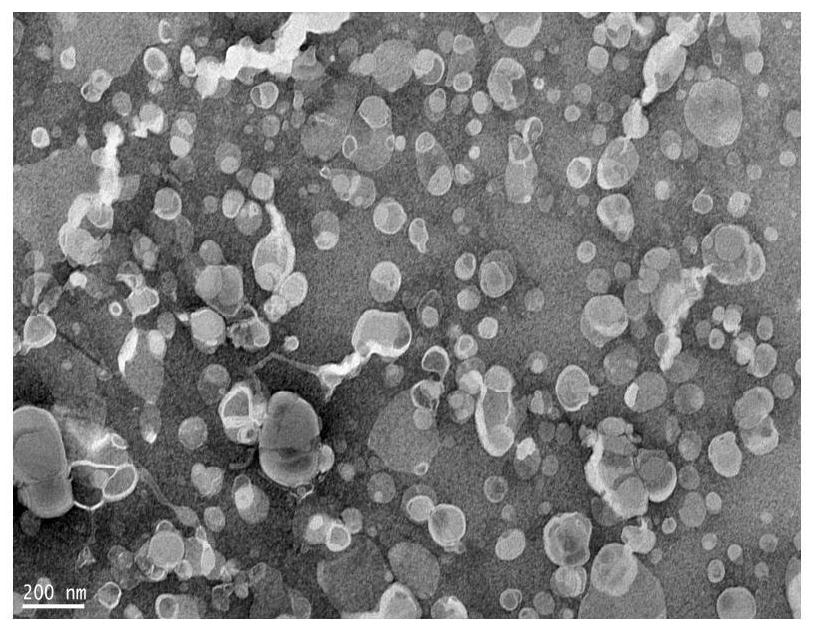
Preparation method of multiple nanoemulsion vaccine adjuvant
A vaccine adjuvant and nanoemulsion technology, applied in the field of vaccine adjuvant preparation, can solve the problems of unsatisfactory stability of ISA206 adjuvant, unreasonable formula ingredients, preparation process, low immunity, etc., to enhance immune cells Proliferation ability, good emulsion stability, good absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A multiple nanoemulsion vaccine adjuvant, which is composed of an inner water phase, an intermediate oil phase and an outer water phase;
[0037] The components in the internal aqueous phase, calculated on the total weight of the water-in-oil-in-water vaccine adjuvant raw materials, are in a proportion of: 50.0 mg / mL lentinan injection 2.0%;
[0038] The components in the intermediate oil phase, based on the total weight of the water-in-oil-in-water vaccine adjuvant raw materials, have the following proportions: 1.2% lecithin, 1.2% ethanol, 2.8% squalene, and 2.8% palm oil;
[0039] The components in the external aqueous phase, based on the total weight of the water-in-oil-in-water vaccine adjuvant raw materials, are in proportions: Tween-80 3.7%, Span-80 3.7%, and the balance is normal saline.
[0040] The preparation method of the above-mentioned multiple nanoemulsion vaccine adjuvant comprises the following steps;
[0041] 1) Dissolving the immune enhancer in physiolo...
Embodiment 2
[0048] A multiple nanoemulsion vaccine adjuvant consisting of an inner water phase, an intermediate oil phase and an outer water phase;
[0049] The components in the internal water phase, based on the total weight of the water-in-oil-in-water type vaccine adjuvant raw materials, the ratio is: 50.0mg / mL lentinan injection 0.5%;
[0050] Each component in the middle oil phase, based on the total weight of the water-in-oil-in-water type vaccine adjuvant raw materials, has a ratio of: 0.8% lecithin, 0.8% ethanol, 3.2% squalene, and 3.2% palm oil;
[0051] Each component in the external water phase, based on the total weight of the water-in-oil-in-water type vaccine adjuvant raw materials, has a ratio of: Tween-80 2.7%, Span-80 2.7%, and the balance is physiological saline.
[0052] The preparation method of the above-mentioned multiple nanoemulsion vaccine adjuvant comprises the following steps;
[0053] 1) Dissolving the immune enhancer in physiological saline, mixing, and ster...
Embodiment 3
[0060] A multiple nanoemulsion vaccine adjuvant consisting of an inner water phase, an intermediate oil phase and an outer water phase;
[0061] Each component in the internal water phase, based on the total weight of the multiple nanoemulsion vaccine adjuvant raw materials, the proportion is: 50.0mg / mL lentinan injection 1.0%;
[0062] Each component in the middle oil phase, based on the total weight of the multiple nanoemulsion vaccine adjuvant raw materials, has a ratio of: 1.0% lecithin, 1.0% ethanol, 3.0% squalene, and 3.0% palm oil;
[0063] Each component in the external water phase, based on the total weight of the multiple nanoemulsion vaccine adjuvant raw materials, has a ratio of: Tween-80 3.2%, Span-80 3.2%, and the balance is physiological saline.
[0064] The preparation method of the above-mentioned water-in-oil-in-water vaccine adjuvant comprises the following steps;
[0065] 1) Fully mix the immune enhancer solution and the inactivated antigen to obtain the i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com