Method for preparing sulfoxide and sulfone through water phase oxidation of thioether
A technology of sulfide and sulfoxide, which is applied in the field of preparing sulfoxide and sulfone by water-phase oxidation of sulfide, can solve the problems of harsh reaction conditions, use of organic solvents, long reaction time and the like, and achieves high product yield, mild reaction conditions, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
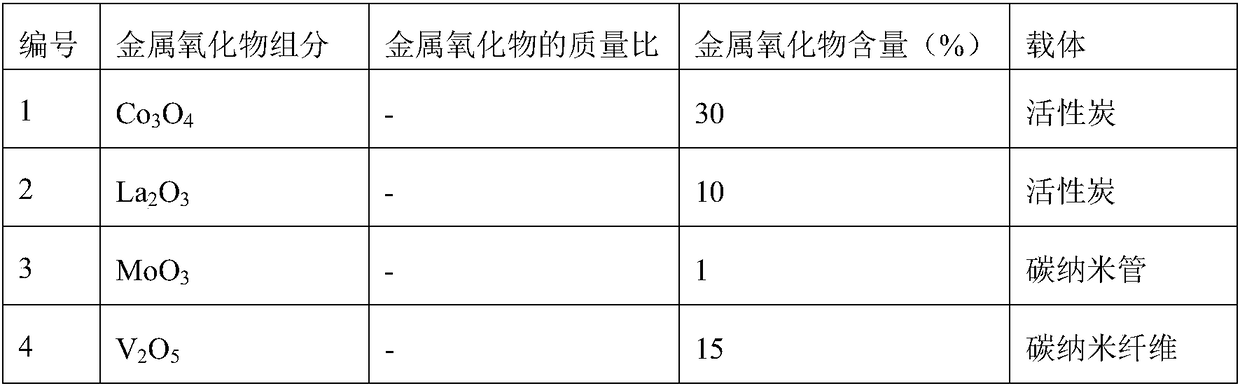
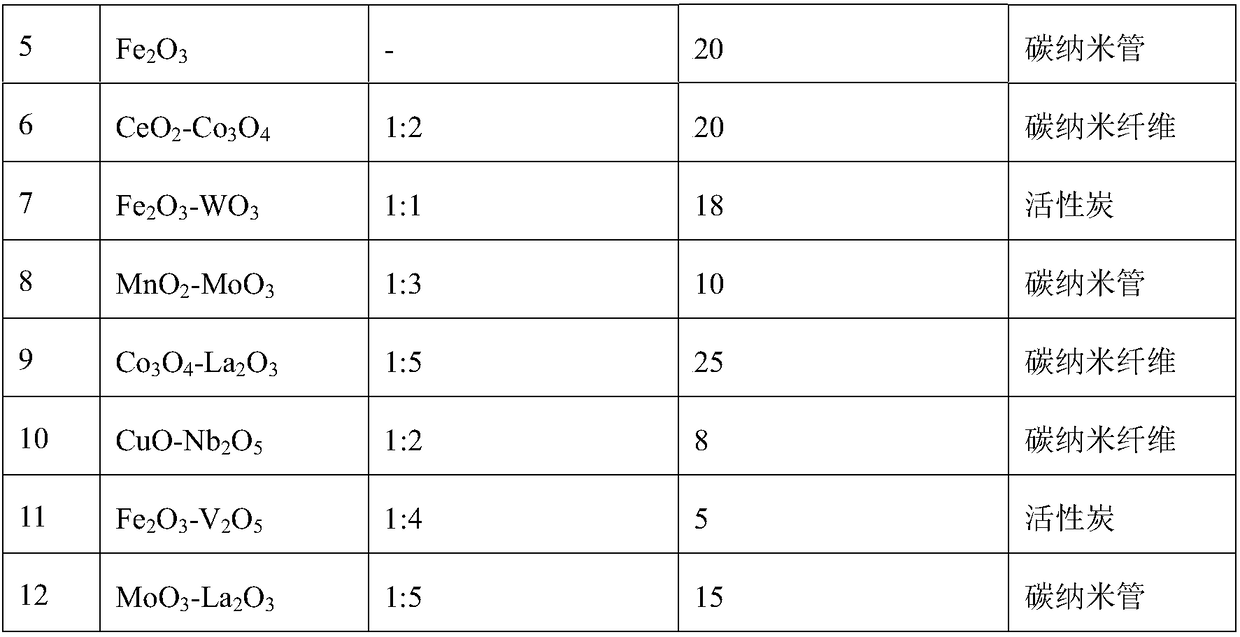
Image
Examples
Embodiment 1
[0033] Embodiment 1 (the catalytic oxidation reaction of diethyl sulfide prepares diethyl sulfoxide):
[0034] The diethyl sulfide aqueous solution of 10wt% is joined in the round bottom flask, then adds catalyst (addition is 5% of diethyl sulfide aqueous solution quality), then adds H 2 o 2 (The amount added is 110% of the molar amount of diethyl sulfide), stirred and mixed evenly, placed in an oil bath, heated to 40° C., and reacted for 60 minutes. After the reaction, samples were taken for gas phase analysis, and the test results are shown in Table 2.
[0035] The catalytic oxidation reaction test result of table 2 diethyl sulfide
[0036] Catalyst number
Embodiment 2
[0037] Embodiment 2 (the catalytic oxidation reaction of ethyl phenyl sulfide prepares ethyl phenyl sulfone):
[0038] The ethyl phenyl sulfide aqueous solution of 20wt% is joined in the round bottom flask, then adds catalyst (addition is 10% of ethyl phenyl sulfide aqueous solution quality), then adds H 2 o 2 (the amount added is 350% of the molar amount of ethyl phenyl sulfide), stirred and mixed evenly, placed in an oil bath, heated to 35° C., and reacted for 150 minutes. After the reaction, samples were taken for gas phase analysis, and the test results are shown in Table 3.
[0039] Catalytic oxidation reaction test result of table 3 ethyl phenyl sulfide
[0040] Catalyst number
Embodiment 3
[0041] Embodiment 3 (the catalytic oxidation reaction of diphenyl sulfide prepares diphenyl sulfoxide):
[0042] The diphenyl sulfide aqueous solution of 5wt% is joined in the round bottom flask, then adds catalyst (addition is 1% of the diphenyl sulfide aqueous solution quality), then adds H 2 o 2 (The amount added is 120% of the molar amount of diphenyl sulfide), stirred and mixed evenly, placed in an oil bath, heated to 35° C., and reacted for 60 minutes. After the reaction, samples were taken for gas phase analysis, and the test results are shown in Table 4.
[0043] The catalytic oxidation reaction test result of table 4 diphenyl sulfide
[0044] Catalyst number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com