Preparation method of optical pure (S)-6-metoxybenzene dihydropyran-3-carboxylic acid
A technology of methoxybenzene and dihydropyran, applied in the field of chiral synthesis, can solve the problems of harsh conditions, high price, harsh reaction conditions and the like, and achieves the effects of mild conditions, low cost and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
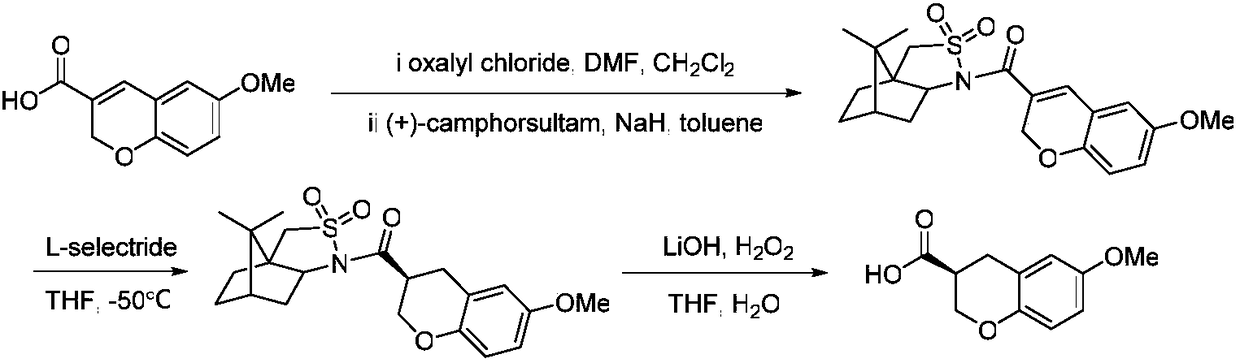
[0044] Raw materials and solvent addition: racemic 6-methoxychroman-3-carboxylic acid (1eq, 1.06g), dissolving agent (1S, 2S)-2-amino-1-(4 -nitrophenyl)propane-1,3-diol (1eq, 1.04g), acetonitrile (80mL), methanol (8mL); the precipitated solid was washed with acetonitrile (50mL) to obtain (S)-6-methoxy 1.01 g of phenylchroman-3-carboxylic acid-(1S,2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diolate. The organic solvents used for extraction were ethyl acetate (50 mL) and water (50 mL). The solvent used for recrystallization was n-hexane (50mL) and acetonitrile (5mL), and finally obtained (S)-6-methoxychroman-3-carboxylic acid 0.37g, the yield was 34.9%, and the optical purity was 95.8%.
[0045] The organic solvent used for extraction when reclaiming the unraveling agent was dichloromethane (80mL), and reclaiming the unraveling agent (1S, 2S)-2-amino-1-(4-nitrophenyl)propane-1,3-di 0.85 g of alcohol, the recovery rate was 81.7%; 0.59 g of (R)-6-methoxychroman-3-carboxylic acid w...
Embodiment 2
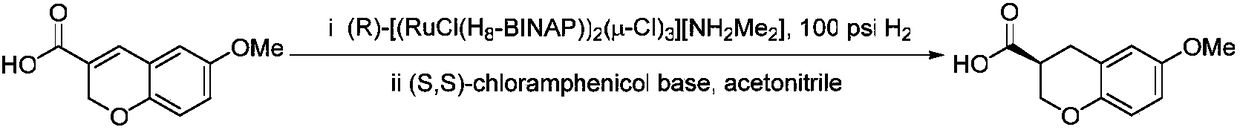
[0047] Raw materials and solvent addition: racemic 6-methoxychroman-3-carboxylic acid (1eq, 3.10g), dissolving agent (1S, 2S)-2-amino-1-(4 -nitrophenyl)propane-1,3-diol (1.2eq, 3.82g), acetonitrile (300mL), methanol (30mL); the precipitated solid was washed with acetonitrile (500mL) to give (S)-6-methanol 3.05 g of oxychroman-3-carboxylic acid-(1S,2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diolate. The organic solvents used for extraction were ethyl acetate (100 mL) and water (100 mL). The solvent used for recrystallization was n-hexane (100mL) and acetonitrile (10mL), and finally obtained (S)-6-methoxychroman-3-carboxylic acid 1.12g, the yield was 36.1%, and the optical purity was 97.1%.
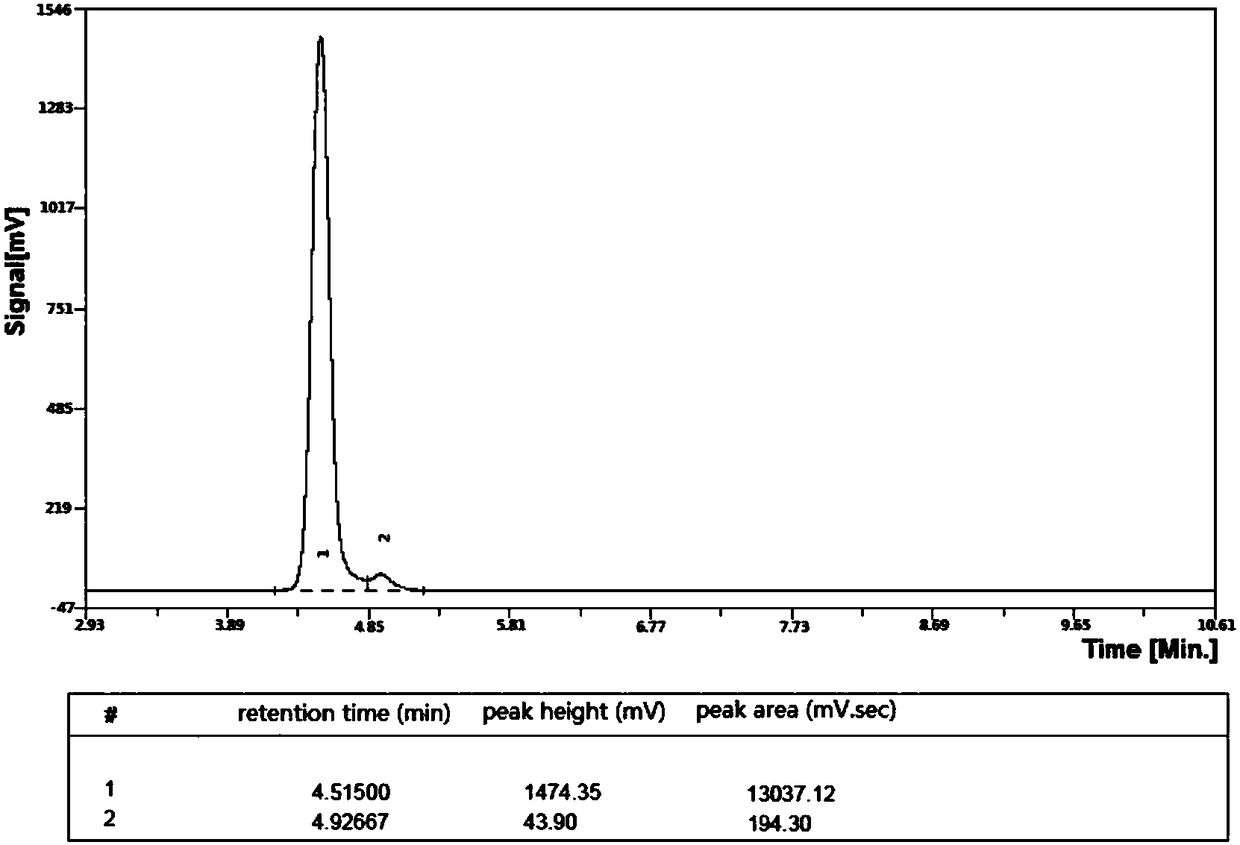
[0048] figure 1 It is the HPLC spectrogram of embodiment 2 gained (S)-6-methoxychroman-3-carboxylic acid; Wherein: mobile phase: n-hexane: Virahol=1:1, injection pressure: 10.0MPa, flow rate: 0.9mL / min, injection time: 10s, injection volume: 10μL.
[0049] The organic solvent used for ex...
Embodiment 3
[0051] Raw materials and solvent addition: racemic 6-methoxychroman-3-carboxylic acid (1eq, 3.10g), dissolving agent (1S, 2S)-2-amino-1-(4 -nitrophenyl)propane-1,3-diol (1.5eq, 4.74g), acetonitrile (300mL), methanol (30mL); the precipitated solid was washed with acetonitrile (500mL) to obtain (S)-6-methanol 3.09 g of oxychroman-3-carboxylic acid-(1S,2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diolate. The organic solvents used for extraction were ethyl acetate (100 mL) and water (100 mL). The solvents used for recrystallization were n-hexane (100 mL) and acetonitrile (10 mL). Finally, 1.14 g of (S)-6-methoxychroman-3-carboxylic acid was obtained, with a yield of 36.8% and an optical purity of 97.3%.
[0052] The organic solvent used in recovery process extraction is dichloromethane (300mL). 3.90 g of the unraveling agent (1S, 2S)-2-amino-1-(4-nitrophenyl)propane-1,3-diol was recovered, the recovery rate was 82.3%, and ( R)-6-methoxychroman-3-carboxylic acid 1.69 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com