Preparation method of palmatine hydrochloride
A technique for palmatine hydrochloride and berberine hydrochloride, which is applied in the field of synthesis of palmatine hydrochloride, can solve the problems of poor feasibility, unsuitability for large-scale production, and pending development of palmatine hydrochloride, and achieve easy operation and large scale Production and economical applicability, beneficial effect on the protection of natural resources and ecological environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
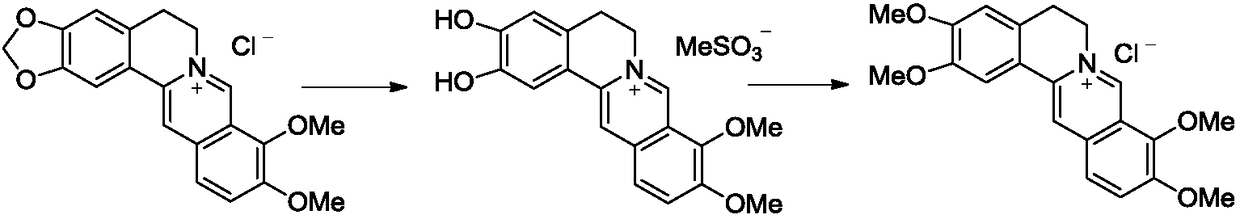
[0018] (1) Weigh 200g of berberine hydrochloride, 1L of toluene and 500g of methanesulfonic acid into a 3L round-bottomed flask equipped with a reflux condenser, thermometer and stirring device. Keep the temperature of the reaction solution at 90°C and stir for about 12 hours. TLC monitors the disappearance of the raw materials, and the stirring is stopped. Add ethanol and stir at reflux for 2 hours. After cooling to room temperature and standing for crystallization, 151 g of 2,3-dihydroxyberberine methanesulfonate was obtained with a yield of 78%.
[0019] (2) Take 100g of the 2,3-dihydroxyberberine methanesulfonate obtained in the above steps and put it into a 3L round bottom flask, add 114g of methyl p-toluenesulfonate and 46g of potassium carbonate, and acetonitrile as the solvent and reflux for 5 hours. TLC monitors the disappearance of the raw materials, and the stirring is stopped. Reduce to room temperature, filter, and wash the filter cake with a small amount of water....
Embodiment 2
[0022] (1) Weigh 200g of berberine hydrochloride, 1L of toluene and 500g of methanesulfonic acid into a 3L round-bottomed flask equipped with reflux condenser, thermometer and stirring device. Keep the temperature of the reaction solution at 90℃. After about 12 hours, TLC Monitor the disappearance of the raw materials and stop stirring. Add ethanol and stir at reflux for 2 hours. After cooling to room temperature and standing for crystallization, 154 g of 2,3-dihydroxyberberine methanesulfonate was obtained with a yield of 80%.
[0023] (2) Take 100g of 2,3-dihydroxyberberine methanesulfonate obtained in the above steps and put it into a 3L round bottom flask, add 600ml of N,N-dimethylformamide to dissolve, add 100ml of dimethyl carbonate and cesium carbonate 109g, after addition, stirred at 140°C for 8 hours, TLC monitored the disappearance of the raw materials, and stopped stirring. It was cooled to room temperature, the solvent was removed, and the precipitated solid was col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com