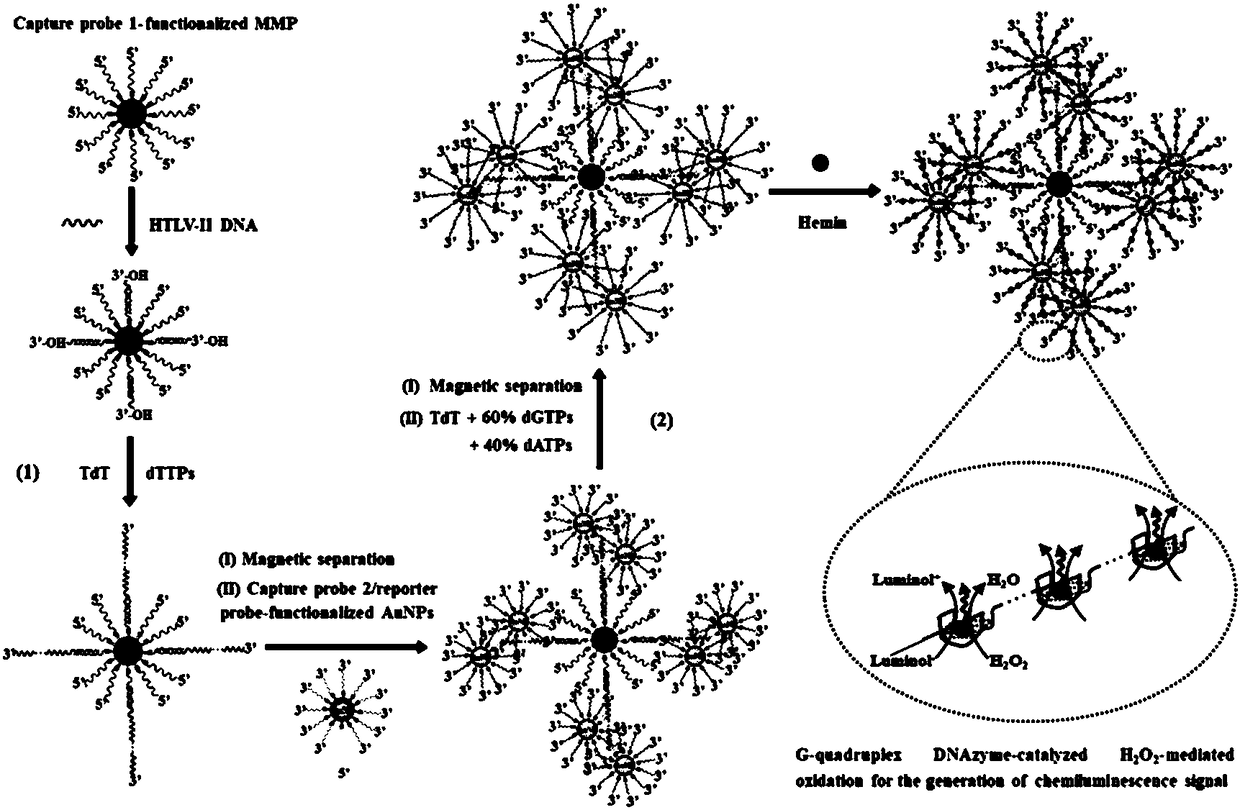
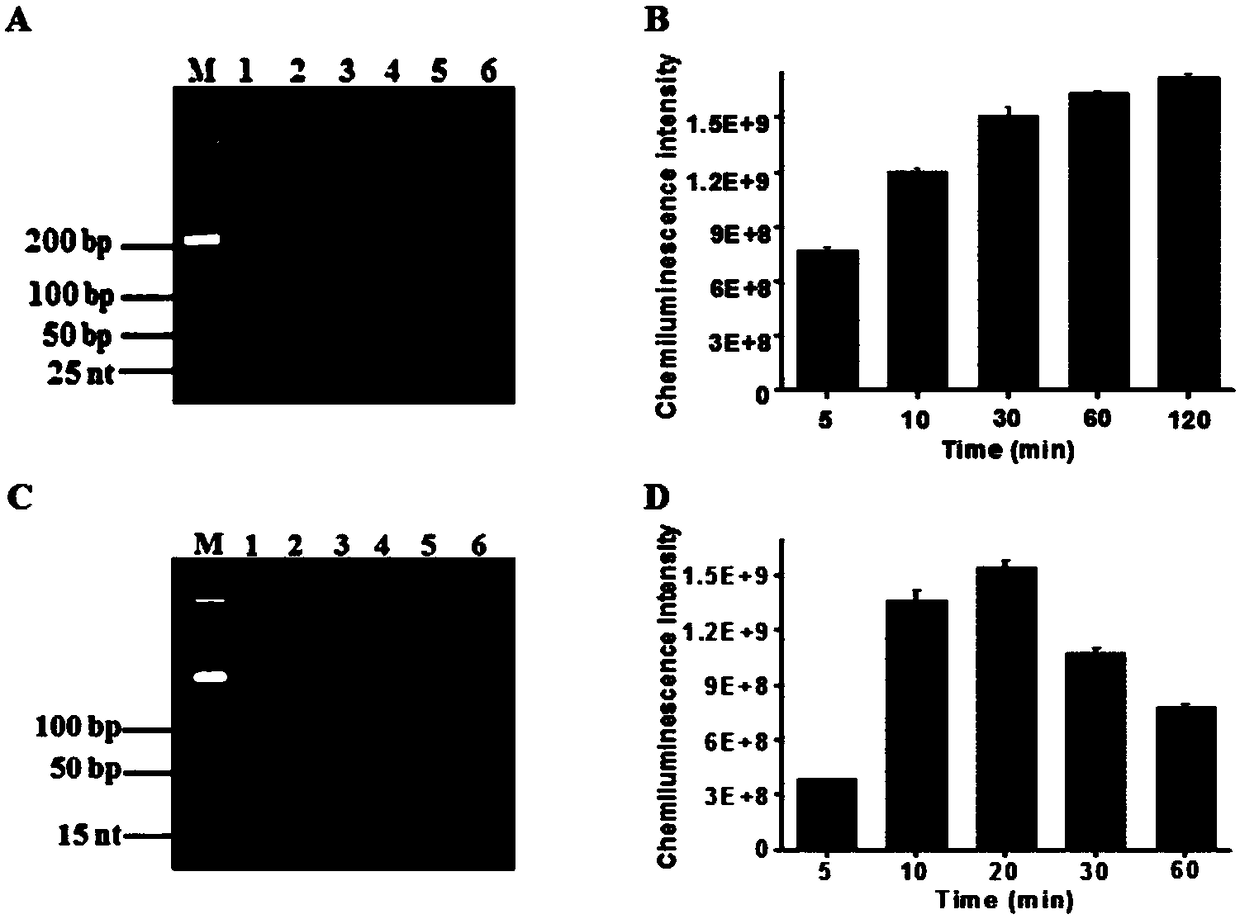
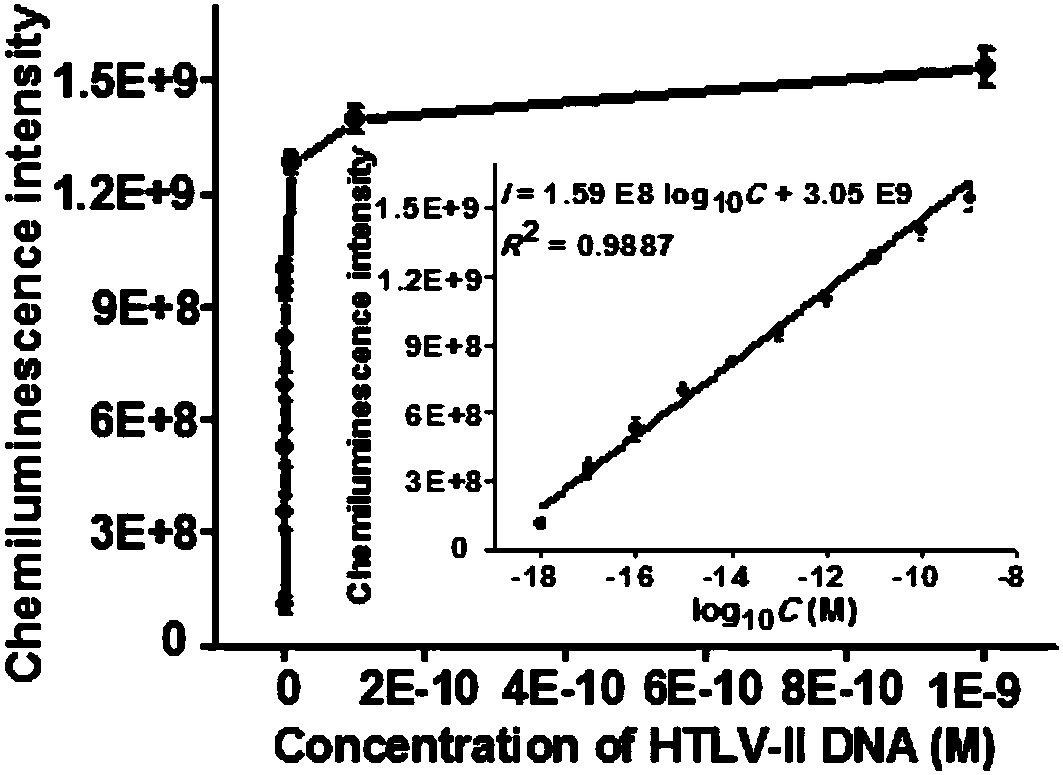
Method for detecting HTLV-II DNA based on enzymatic catalysis controllable self-assembly bio-barcode
A biological barcode and self-assembly technology, applied in the field of chemiluminescence, to achieve the effects of improved detection accuracy, good technical effect, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] In a preferred embodiment, the preparation method of magnetic microspheres in the above-mentioned step (1) comprises the following steps:
[0049] Dissolve capture probe 1 in 1×Tris-EDTA buffer to prepare a stock solution; add 10 μmol / L capture probe 1 and 10 mg / mL streptavidin-coated magnetic microspheres to ultrapure water , and incubated at room temperature for 10 min to bind the capture probe 1 to the streptavidin-coated magnetic microspheres; use magnetic separation to remove excess capture probe 1 in the solution to obtain a functionalized capture probe 1 magnetic microspheres coated with streptavidin.
[0050] The ratio of capture probe 1: magnetic microspheres: ultrapure aqueous solution is 1 μL: 1 μL: 10 μL.
[0051] Preferably, the method for preparing AuNPs in the above step (2) includes the following steps: dissolving the capture probe 2 and the reporter probe in 1×Tris-EDTA buffer to prepare a stock solution; mixing 0.5 mg / mL of AuNPs, Add 1 μmol / L captur...
Embodiment 1
[0058] Terminal deoxynucleotidyl transferase catalyzes the self-assembly of biological barcodes to achieve dendritic chemiluminescent signal amplification: terminal deoxynucleotidyl transferase needs to perform two polymerization extension reactions. In the first polymerization extension reaction, different concentrations of the target substance HTLV-II DNA was added to 20 μL hybridization solution containing freshly prepared capture probe 1 functionalized magnetic microspheres, 750 mmol / L NaCl and 75 mmol / L sodium citrate, and incubated at room temperature for 10 minutes to form 3'- Double-stranded DNA with overhanging hydroxyl ends. After magnetic separation, add 0.4 units of terminal deoxynucleotidyl transferase to 20 μL of the polymerization reaction solution composed of the separated precipitate product, including 2 μL of 100 μmol / L dTTP, 2 μL of 10× terminal deoxynucleotidyl transferase reaction buffer, 2 μL 2.5mmol / L CoCl 2 , and at 37°C, the protruding 3' terminal HTL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com