Preparation method and application of a magnetically recyclable Fe-mof photocatalyst
A photocatalyst and magnetic recovery technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of cost suppression and insufficient photocatalytic performance of MOFs In order to achieve the effects of easy recycling, excellent visible light catalytic activity and magnetic recyclability, and low raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] MOF photocatalyst [Fe(2-mim) 3· nH 2 The preparation method of O], the steps are as follows:
[0045] (1) 40mmol of organic ligand 2-MI was dissolved in 50ml methanol solution, and after mixing and stirring for about 30 minutes, a uniform methanol solution of 2-MI was obtained;
[0046] (2) 4mmol Fe(NO 3 ) 3 ·9H 2 O was dissolved in 50ml methanol solution, mixed and stirred to obtain uniform Fe 3+ solution, the uniform methanol solution of 2-MI obtained in step (1) was added dropwise to Fe 3+ In the solution, the stirring reaction was performed for about 60 minutes to obtain a suspension;
[0047] (3) After the reaction product obtained in step (2) was allowed to stand at room temperature for more than 20 hours, washed with ethanol and water respectively, and then, the washed reaction product was dried under static vacuum at 80°C for 10 hours, and finally Obtained [Fe(2-mim) 3· nH 2 O] photocatalyst.
[0048] figure 1 is [Fe(2-mim) 3· nH 2 O] Image taken un...
Embodiment 2
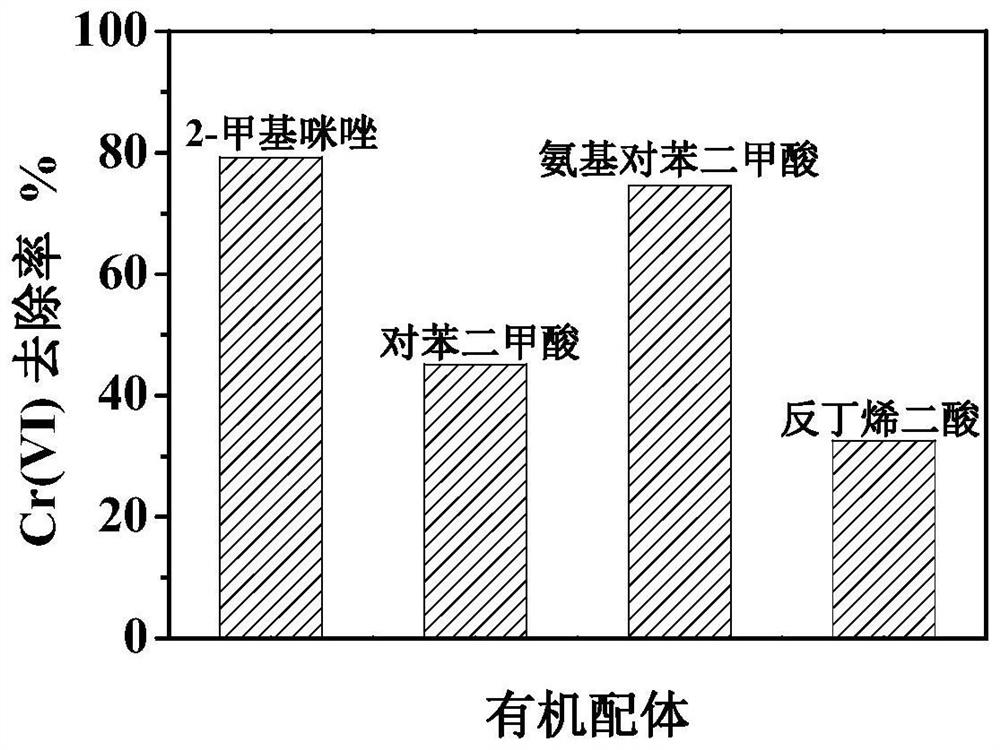
[0050] Different organic ligands and Fe 3+ Different MOFs with Fe as the central atom can be formed. Using the reported method, by changing the organic ligands, H 2 BDC, NH 2 -H 2 MOFs were prepared with BDC and fumaric acid as organic ligands with ferric nitrate (nonahydrate).
[0051] Taking the degradation of chromium-containing Cr(VI) wastewater as an example to test the catalyst activity, 100 mg of Fe-based MOF visible light catalysts prepared in different proportions were added to the reactor with a volume of 100 mL and a concentration of 80 μmol / L chromium-containing Cr(VI) wastewater. , do not adjust the pH, continue to stir, stir in the dark for 30min to the adsorption equilibrium, turn on the visible light source, and take samples at 15min intervals.
[0052] like figure 2 , the Fe-based MOF with 2-MI as the organic ligand prepared by the present invention has the best visible light degradation Cr(VI) effect, and is the only one with magnetic properties.
Embodiment 3
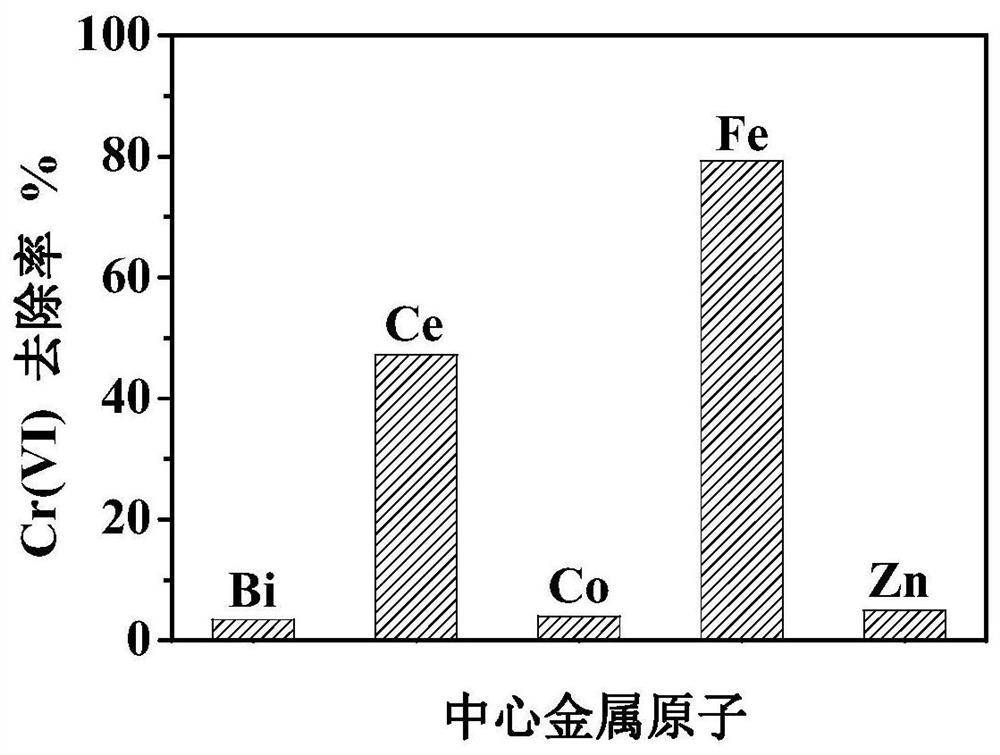
[0054] Different central metal atoms and organic ligands 2-MI can form MOFs with different central metal atoms. Take M(NO 3 ) X (where M=Ce, Co, Bi, Fe, Zn) provided the central metal atom, and using methanol as the solvent, a series of MOFs with different central metal atoms were prepared by a one-step method similar to the preparation of Fe-MOF. And compare according to the chromium reduction operation of Example 2.
[0055] like image 3 , the Fe-based MOF with Fe as the central metal atom prepared by the present invention has the best visible light degradation Cr(VI) effect, and is the only one with magnetic properties.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com