Ferulic acid-cyclodextrin covalent coupling compound and its preparation method and application
A technology of covalent coupling and cyclodextrin, which is applied in the fields of medicine and food, can solve the problems of complex properties such as instability, loss, and damage of intermolecular forces, so as to retain antioxidant and biological activity and link stability , The effect of water solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
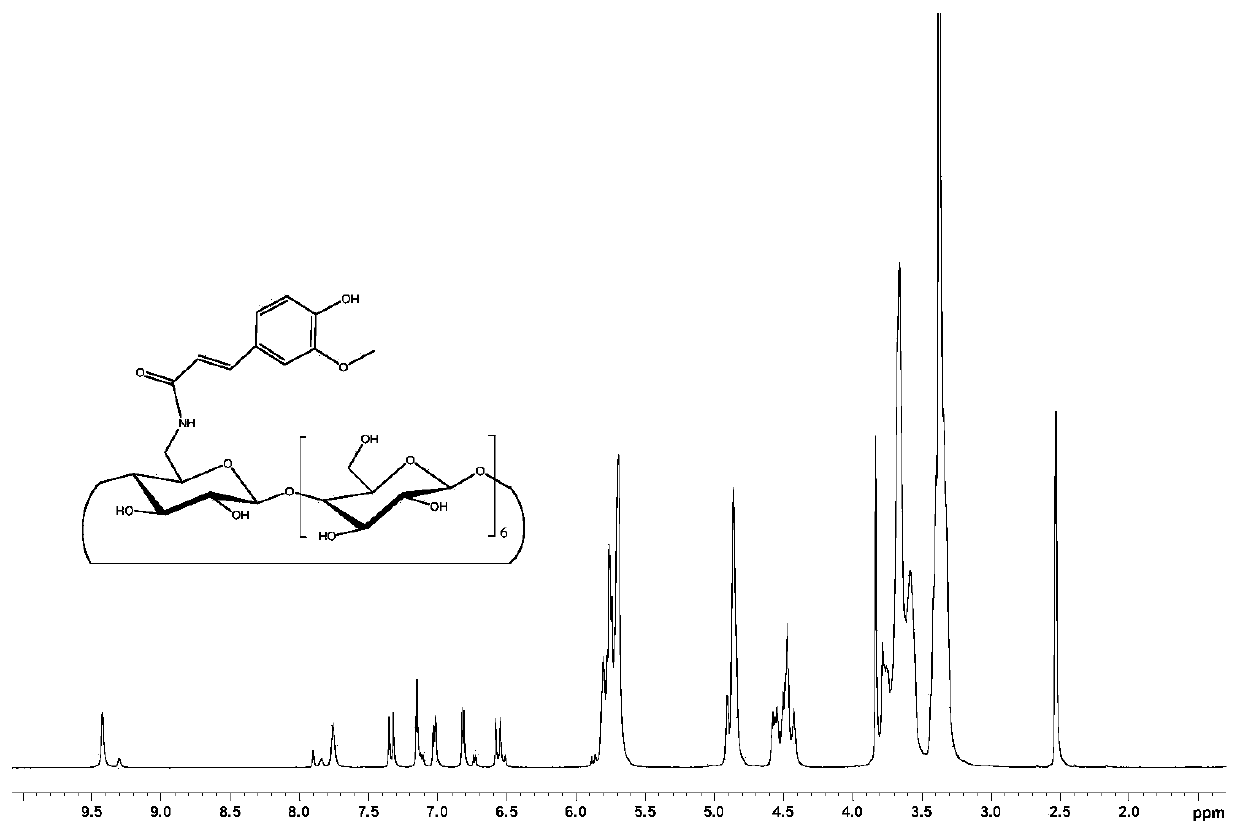
[0059] Embodiment 1, the synthesis of the ferulic acid-cyclodextrin covalent coupling compound FA-CD-(I) connected by amide bond
[0060] β-cyclodextrin (20g, 17.6mmol) was suspended in 250mL of distilled water, and 10mL of aqueous solution containing NaOH (2.19g, 54.75mmol) was slowly added dropwise to the suspension using a constant pressure dropping funnel, and the whole process was carried out under ice bath conditions. After the solution was clarified after the dropwise addition, p-toluenesulfonyl chloride (5.04 g, 26.46 mmol) was dissolved in 15 mL of acetonitrile, and added dropwise to the above reaction system. After the system was reacted at room temperature for 2 hours, the precipitate was removed by filtration. The filtrate was adjusted to pH 6 with dilute hydrochloric acid, placed in a refrigerator at 4°C overnight, and the precipitate was collected by centrifugation. The precipitate was recrystallized twice in water, and the product mono-6-p-toluenesulfonyl-β-cyc...
Embodiment 2
[0068] Embodiment 2, the synthesis of conjugated product FA-CD (II)
[0069] The preparation method of mono-6-p-toluenesulfonyl-β-cyclodextrin is shown in Example 1.
[0070] Add mono-6-p-toluenesulfonyl-β-cyclodextrin (2.5g, 1.94mmol) into a round-bottomed flask containing 15mL of ethylenediamine, and react at 70°C for 8 hours under nitrogen protection. After the reaction, cool After reaching room temperature, the reaction solution was poured into a large amount of acetone to precipitate a precipitate, which was filtered and dried to obtain the product mono-6-ethylenediamine-β-cyclodextrin.
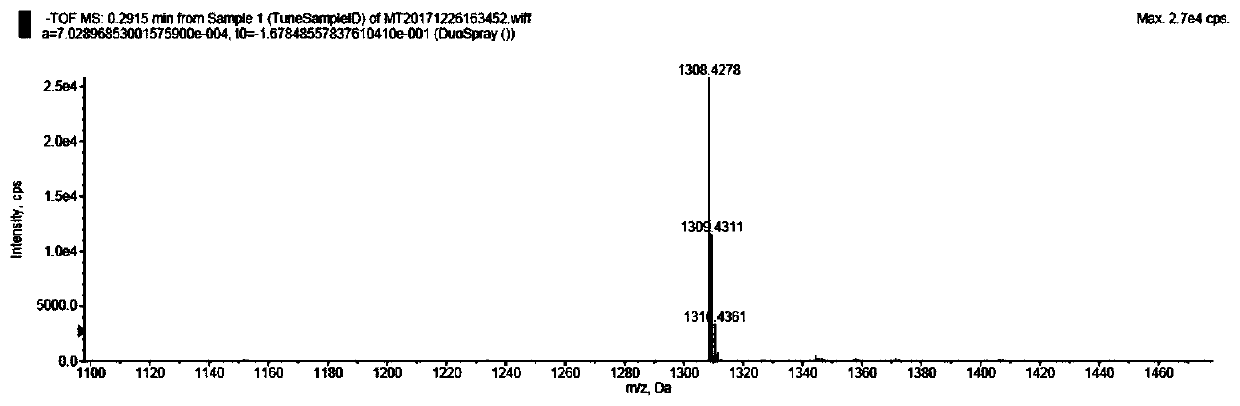
[0071] To a round bottom flask containing 20 mL of anhydrous DMF was added mono-6-ethylenediamine-β-cyclodextrin (1.177 g, 1 mmol), ferulic acid (0.233 g, 1.2 mmol), EDCI (0.575 g, 3 mmol) and HOBT (0.135g, 1mmol), stirred and reacted under ice bath for half an hour, then warmed up to room temperature, and stirred under nitrogen protection for 36h. After the reaction is complete, pour ...
Embodiment 3
[0075] Embodiment 3, the synthesis of conjugated product FA-CD (Ⅲ)
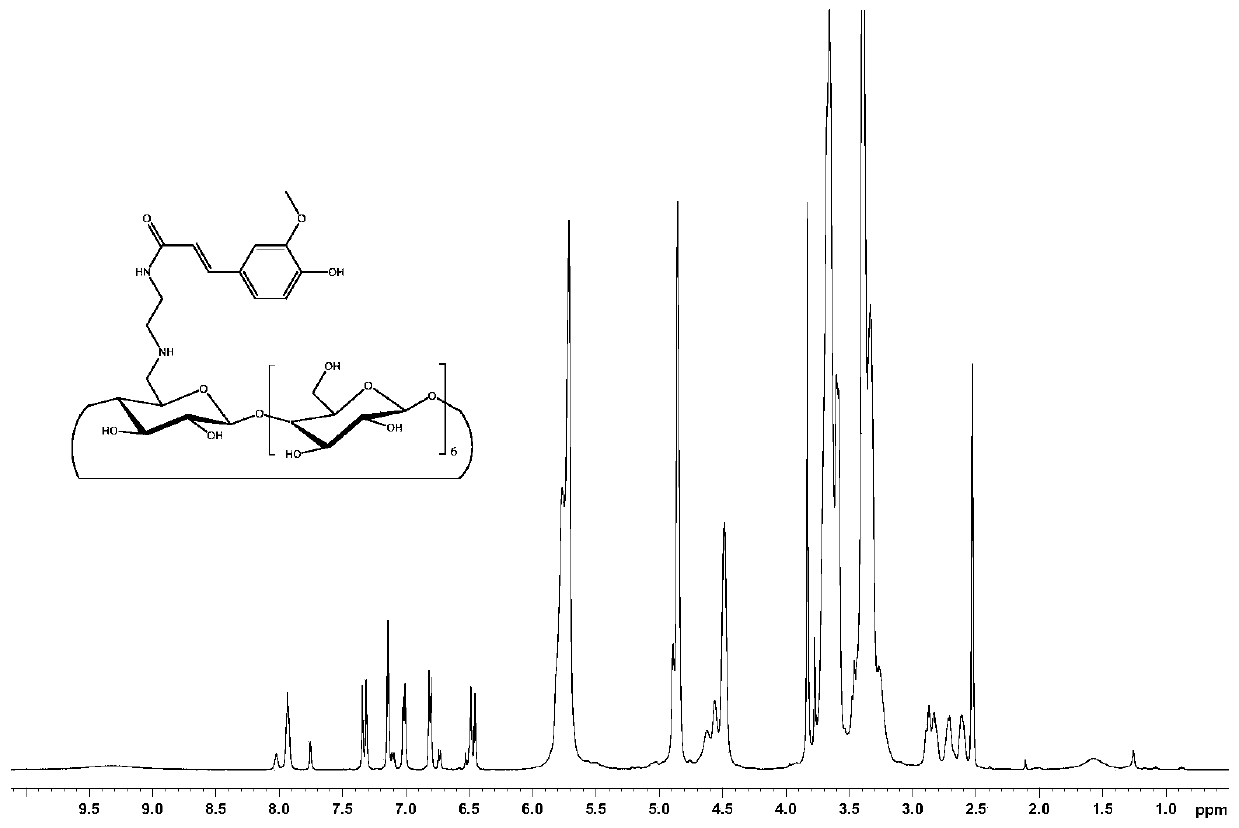
[0076]Take mono-6-p-toluenesulfonyl-β-cyclodextrin (2.0g, 1.55mmol) into a round-bottomed flask containing 8g of hexamethylenediamine, and react at 80°C for 8 hours under nitrogen protection. After the reaction, cool After reaching room temperature, the reaction solution was poured into a large amount of acetone to precipitate a precipitate, which was filtered and dried to obtain the product mono-6-hexamethylenediamine-β-cyclodextrin.
[0077] To a round bottom flask containing 20 mL of anhydrous DMF was added mono-6-hexanediamine-β-cyclodextrin (1.233 g, 1 mmol), ferulic acid (0.233 g, 1.2 mmol), EDCI (0.575 g, 3 mmol) and HOBT (0.135g, 1mmol), stirred under ice bath for half an hour, then raised to room temperature and stirred for 36h, after the reaction was completed, poured the reaction solution into 300mL acetone, precipitated, filtered, and dissolved the precipitate in a small amount of water and methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com