A kind of external use triptolide lipid nanoparticle and preparation method
A nanotechnology of triptolide and solid lipid, which is applied in the field of medicine, can solve the problems of organic solvent residue and high equipment requirements, and achieve the effect of high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
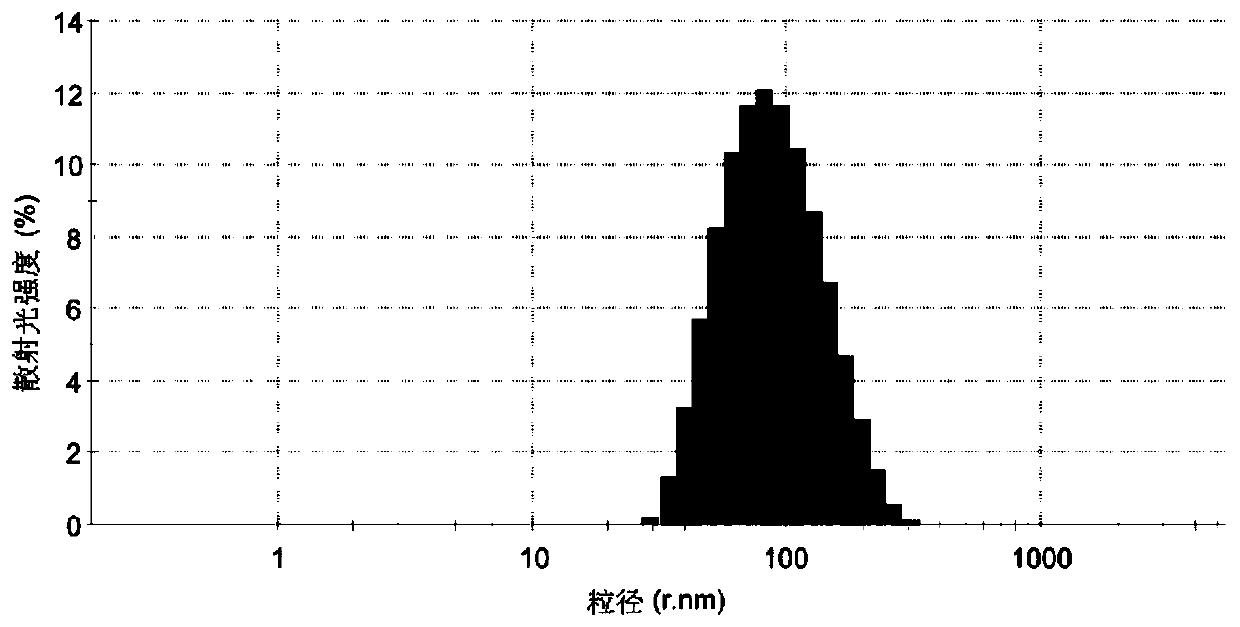
[0092] Mix triptolide 7.5mg, stearic acid 75mg, TPGS 150mg, Transcutol HP 75mg at 80°C, -1 Stir for 10 minutes under the same conditions to form the oil phase;
[0093] Heat 20ml of deionized water to the same temperature as the oil phase, and add it dropwise to the oil phase to make the system appear uniform and transparent, and continue to stir for 10 minutes to obtain triptolide solid lipid nanoparticle colostrum;
[0094] Rapidly disperse the above colostrum into 20 times the volume of colostrum in ice water at a temperature of 0±2° C., stir and solidify for 10 minutes to obtain triptolide solid lipid nanoparticles.
Embodiment 2
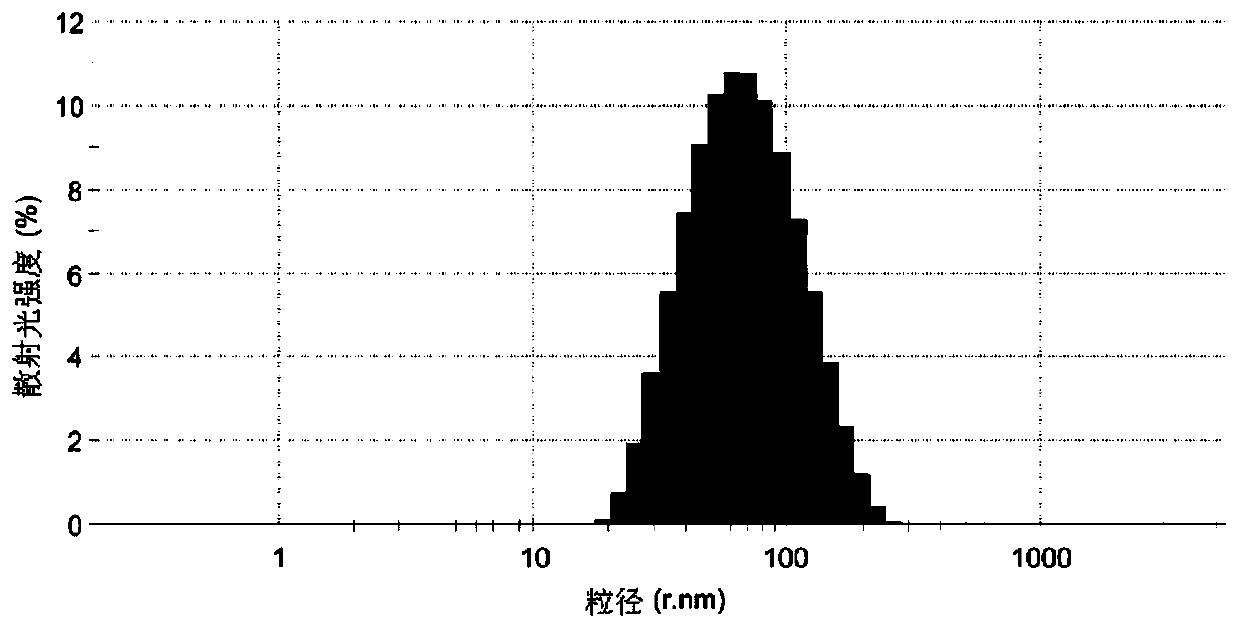
[0096] Mix triptolide 7.5mg, glyceryl behenate 80mg, TPGS 150mg, Transcutol HP75mg at 80°C, -1 Stir for 10 minutes under the same conditions to form the oil phase;
[0097] Heat 25ml of deionized water to the same temperature as the oil phase, and add it dropwise to the oil phase to make the system appear uniform and transparent, and continue to stir for 10 minutes to obtain triptolide solid lipid nanoparticle colostrum;
[0098] The above-mentioned colostrum is rapidly dispersed into 15 times the volume of the colostrum in ice water at a temperature of 0±2° C., stirred and solidified for 10 minutes to obtain triptolide solid lipid nanoparticles.
Embodiment 3
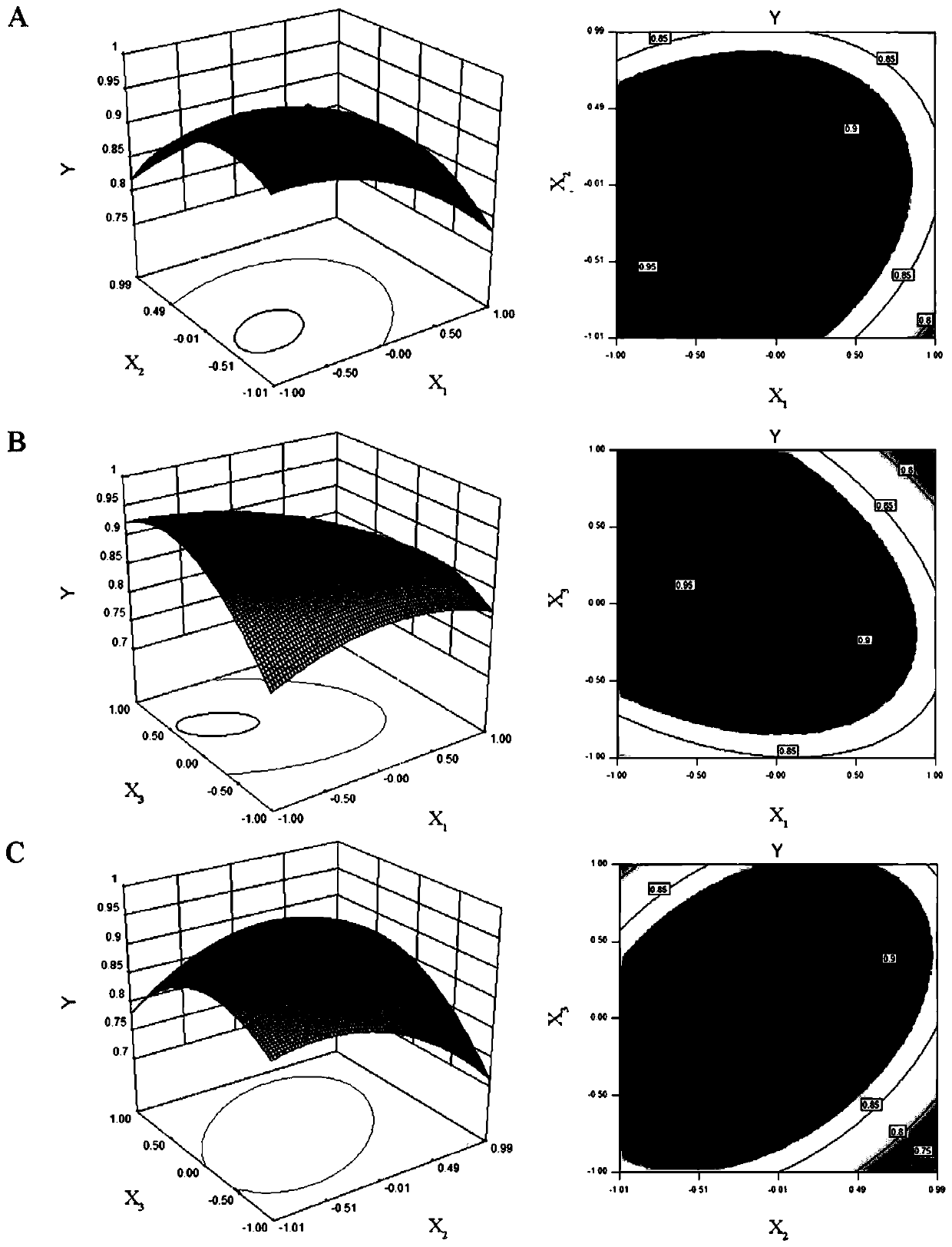
[0100] Mix triptolide 6.3mg, glyceryl behenate 75mg, Tween 80 150mg, Transcutol HP 75mg at 90°C, -1 Stir for 10 minutes under the same conditions to form the oil phase;
[0101] Heat 15ml of deionized water to the same temperature as the oil phase, and add it dropwise to the oil phase to make the system appear uniform and transparent, and continue stirring for 10 minutes to obtain triptolide solid lipid nanoparticle colostrum;
[0102] Rapidly disperse the above colostrum into 20 times the volume of colostrum in ice water at a temperature of 0±2° C., stir and solidify for 10 minutes to obtain triptolide solid lipid nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com