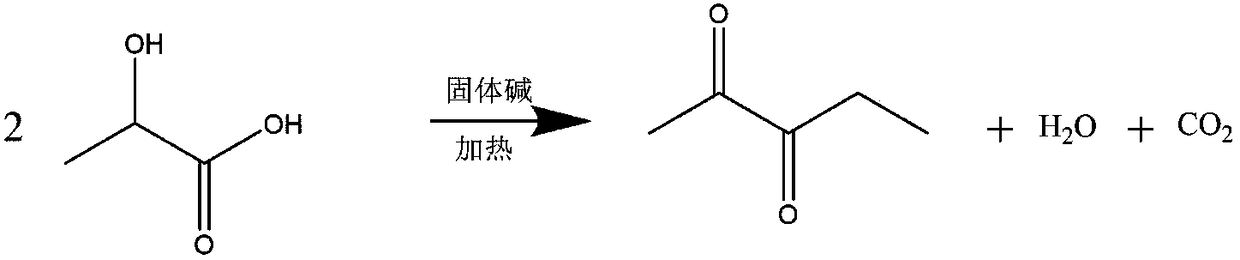
Synthetic method of 2,3-pentanedione
A synthesis method and pentanedione technology are applied in chemical instruments and methods, condensation preparation of carbonyl compounds, organic chemistry, etc., and can solve the problems of complicated catalyst preparation, difficult operation control, industrial application limitations, etc., and achieve high selectivity and Conversion rate, simple production operation, yield reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of solid base
[0023] Weigh 2.9g KF, add it to the beaker, add 20ml water, stir, then weigh 6.2g Al 2 o 3 Added to the above solution (KF and Al 2 o 3 The molar ratio is 1:1.2), heated to 60°C and stirred for 1 hour to obtain a mixture, and put the obtained mixture into a vacuum drying oven at 50-60°C for 12 hours to obtain an unactivated solid superbase catalyst. The above-mentioned solid is activated for 4 hours at 350-550° C. under a nitrogen atmosphere to obtain an activated solid superbase catalyst.
[0024] (2) Catalytic synthesis of 2,3-pentanedione
[0025] Mix 75g of lactic acid solution (90% content) with 5g of the above-prepared solid base catalyst, and slowly heat the reaction mixture. When the reaction temperature reaches 200°C, the lactic acid undergoes condensation dehydration and decarboxylation to generate 2,3-pentanedione. At the same time, the generated 2,3-pentanedione and water are vaporized from the reaction mixture system, an...
Embodiment 2
[0027] (1) Preparation of solid base
[0028] Weigh 2.9g KF, add it into the beaker, add 20ml water, stir, then weigh 10.2gAl 2 o 3 Added to the above solution (KF and Al 2 o 3 The molar ratio is 1:2), heated to 60°C and stirred for 2h to obtain a mixture, and put the obtained mixture into a vacuum drying oven at 50-60°C for 12h to obtain an unactivated solid superbase catalyst. The above-mentioned solid is activated for 5 hours at 350-550° C. under a nitrogen atmosphere to obtain an activated solid superbase catalyst.
[0029] (2) Catalytic synthesis of 2,3-pentanedione
[0030] Mix 75g of lactic acid solution (90% content) with 5g of the above-prepared solid base catalyst, and slowly heat the reaction mixture. When the reaction temperature reaches 200°C, the lactic acid undergoes condensation dehydration and decarboxylation to generate 2,3-pentanedione. At the same time, the generated 2,3-pentanedione and water are vaporized from the reaction mixture system, and after b...
Embodiment 3
[0032] (1) Preparation of solid base
[0033] Weigh 6.9gK 2 CO 3 , into the beaker, add 20ml of water, stir, and weigh 6.2g of Al 2 o 3 Added to the above solution (K 2 CO 3 and Al 2 o 3 The molar ratio is 1:1.2), heated to 60°C and stirred for 1.5h to obtain a mixture, and put the obtained mixture into a vacuum drying oven at 50-60°C for 12h to obtain an unactivated solid superbase catalyst. The above-mentioned solid is activated for 4.5 hours at 350-550° C. under a nitrogen atmosphere to obtain an activated solid superbase catalyst.
[0034] (2) Catalytic synthesis of 2,3-pentanedione
[0035] Mix 75g of lactic acid solution (90% content) with 5g of the above-prepared solid base catalyst, and slowly heat the reaction mixture. When the reaction temperature reaches 200°C, the lactic acid undergoes condensation dehydration and decarboxylation to generate 2,3-pentanedione. At the same time, the generated 2,3-pentanedione and water are vaporized from the reaction mixture s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com