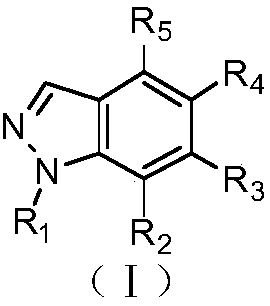
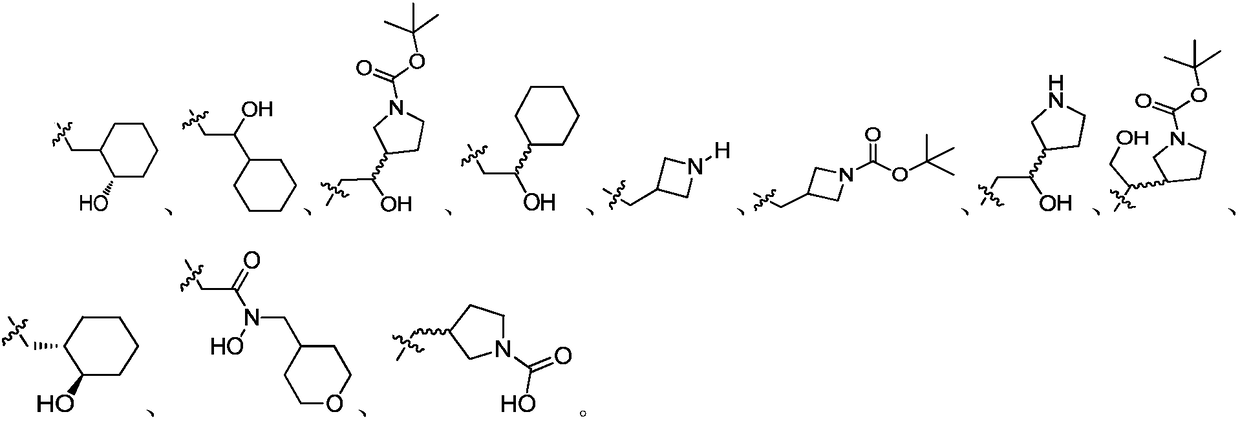
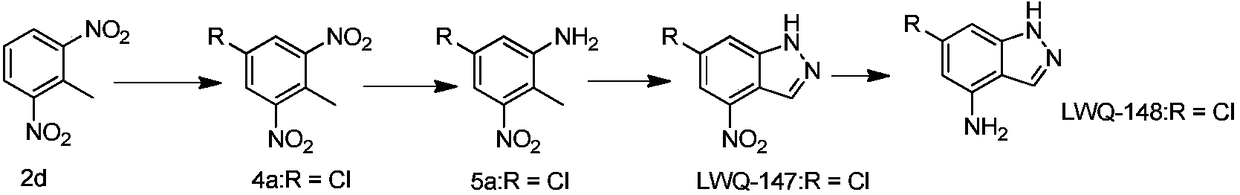
Polysubstituted-indazole compounds and application of same as IDO inhibitors
A compound and mixture technology, applied in the field of IDO inhibitor, can solve the problems of reducing tryptophan concentration, stagnation of synthesis, inhibiting killing effect, etc., and achieving excellent inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Synthesis of Compounds LWQ-136, LWQ-138, LWQ-156, LWQ-167, LWQ-223
[0072] The synthetic route is as follows:
[0073]
[0074] 1 Synthesis of raw material LWQ-221:
[0075] Take a dry 50mL pear-shaped bottle, add 2-methyl-1,3-dinitrobenzene (CAS: 606-20-2, 5.00g, 27.45mmol, purchased from Chengdu Ruioke Reagent Company) with 30mL concentrated sulfuric acid Dissolve, slowly add 1,3-dibromo-5,5-dimethylhydantoin (CAS: 77-48-5, 4.29g, 15.00mmol, purchased from Chengdu Ruioke Reagent Co., Ltd.) under stirring in an ice bath, drop After the addition was completed, the reaction was stirred at room temperature for 15 hours. TLC showed that the reaction of the raw materials was complete. The reaction solution was slowly poured into ice water, filtered, and the filter cake was vacuum-dried to obtain white solid powder 5-bromo-2methyl-1,3-dinitrobenzene (6.59 g, yield 92%).
[0076] Take a dry 50mL pear-shaped flask, dissolve 5-bromo-2methyl-1,3-dinitrobenzene (...
Embodiment 2
[0094] Example 2 Synthesis of Compounds LWQ-154, LWQ-155, LWQ-158, LWQ-159, LWQ-165, LWQ-166
[0095] The synthetic route is as follows:
[0096]
[0097] 1 Synthesis of intermediate 37b
[0098] Under the protection of Ar gas, triphenylphosphine methyl bromide (5.34 g, 15.06 mmol) was dissolved in redistilled THF and cooled to -78°C. Slowly add 2.5M n-butyllithium (6.00mL, 15.06mmol) dropwise, after the addition is complete, stir at the same temperature for 1h, add a THF solution of compound 36 (2.00g, 10.04mmol), stir for 1h, and gradually rise to 0°C. Add saturated ammonium chloride aqueous solution to stop the reaction, spin the reaction solution to dryness, add water to dilute the residue, extract with EA three times, wash with saturated sodium chloride aqueous solution twice, dry, and concentrate. The crude product was purified by column chromatography (PE:EA=20:1) to obtain compound 37b (600 g, 3.04 mmol) as a colorless oily liquid with a yield of 30%.
[0099] 2 ...
Embodiment 3
[0114] The synthesis of embodiment 3 compound LWQ-162, LWQ-163
[0115] The synthetic route is as follows:
[0116]
[0117] 1 Preparation of intermediate 40
[0118] Dissolve 3-azetidinecarboxylic acid 39 (2.47g, 24.46mmol) in 30mL methanol, slowly add SOCl dropwise at 0°C 2 (8.87 mL, 122.3 mmol). After addition, warm to room temperature and stir for 10h. The reaction solution was spin-dried, and the SOCl in the residue was removed with dichloromethane. 2 , and spun 3 times, the crude product was a colorless viscous liquid. Because the water solubility of compound 40 was very good, no further post-treatment was carried out, and the yield was quantified.
[0119] 2 Preparation of intermediate 41
[0120] Compound 40 (3.68g, 24.46mmol) was dissolved in acetonitrile, followed by adding Et 3 N (3.39mL, 24.46mmol), (Boc) 2 O (5.87 g, 26.91 mmol) in acetonitrile and DMAP (0.30 g, 2.45 mmol). After addition, stir at room temperature for 5h. TLC detected that the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com