Application of short peptide to preparation of immunoregulation medicament
A short peptide and drug technology, applied in the field of biomedicine, can solve the problems of poor stability and shortened half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1, miPEP155 sequence analysis and in vitro synthesis
[0080] 1. miPEP155 sequence analysis
[0081] The sequence of miR-155 host gene is as follows:
[0082]
[0083]
[0084] In the above sequence, the underlined part in italics is the predicted sequence of miPEP155. The sequence translated into amino acid is: MEMALMVAQTRKGKSVV (SEQ ID NO: 2).
[0085] 2. In vitro synthesis of miPEP155
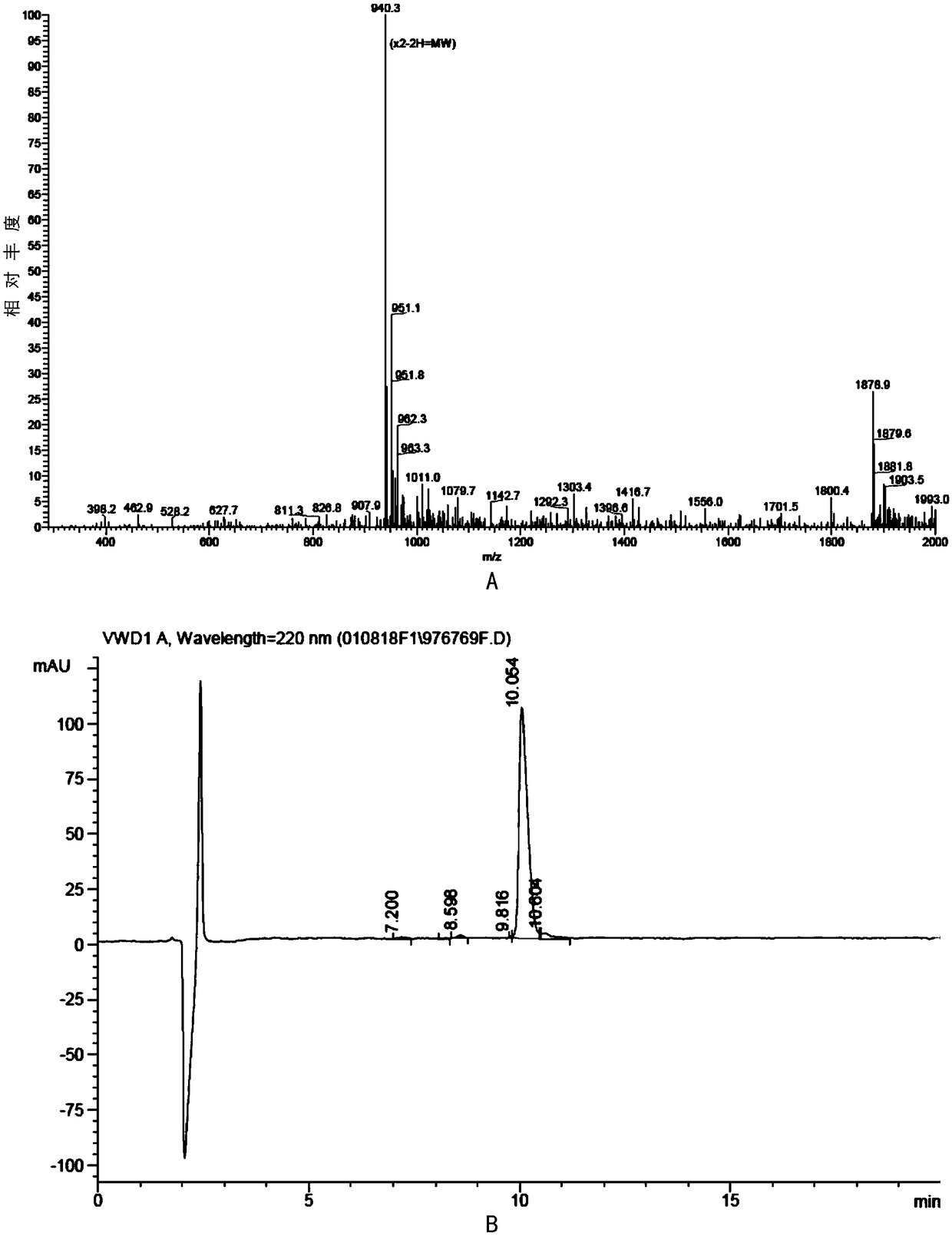
[0086] Using the conventional solid-phase peptide synthesis method, the peptide was synthesized according to the amino acid sequence of SEQ ID NO: 2, and the mass spectrometry analysis determined that the amino acid was correct as figure 1 A (miPEP155). The purity is 95.1%, as figure 1 b. Dissolve in ddH before use 2 O Standby.
Embodiment 2
[0087] Example 2, expression of miPEP155 in cells
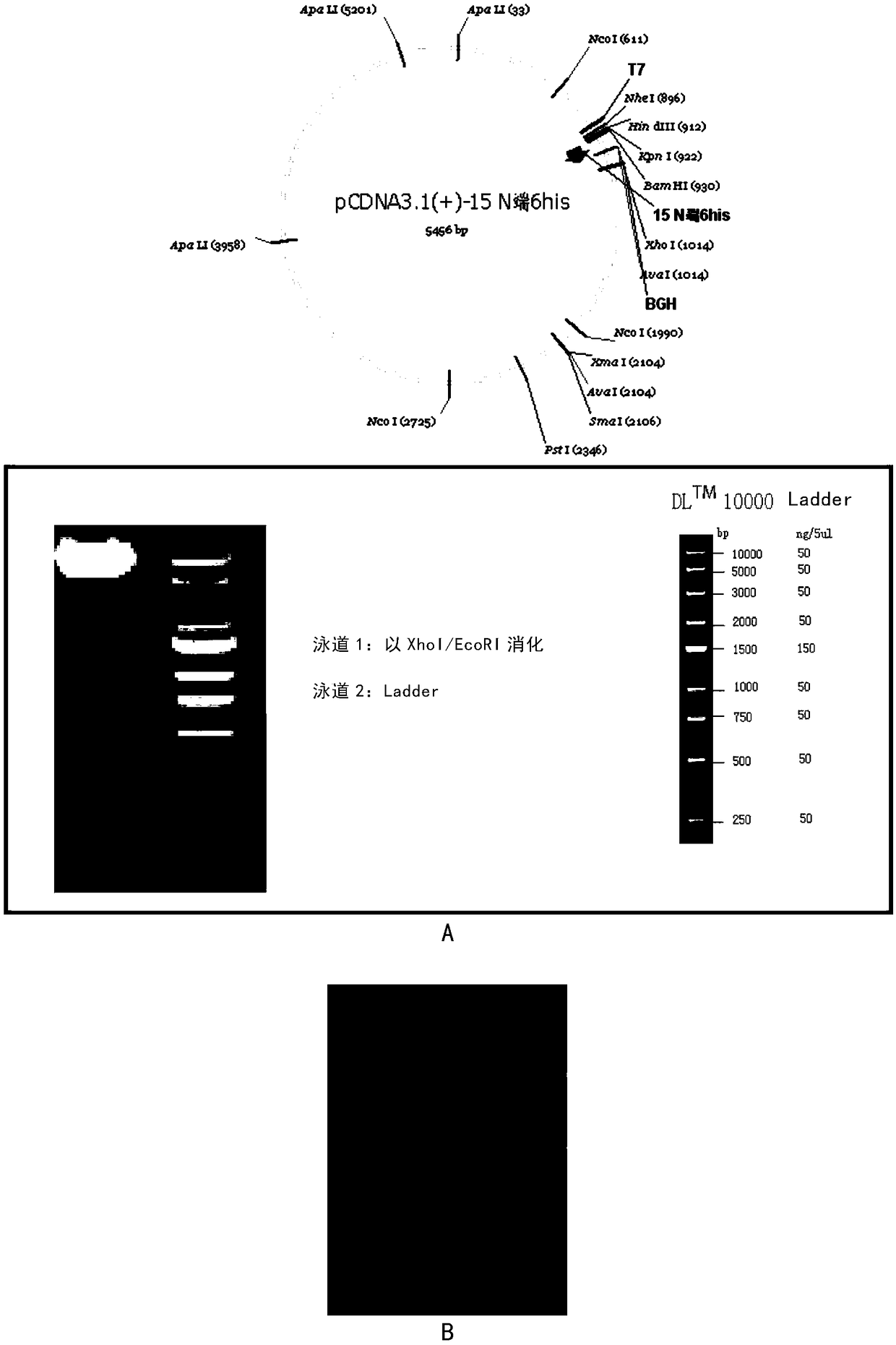
[0088] The coding sequence of miPEP155 was constructed into the BamHI / XhoI restriction site of plasmid pcDNA3.1(+). Thus, the obtained recombinant plasmid can form the fusion protein of miPEP155 and his-tag after expression, and the sequence of the recombinant plasmid is confirmed to be correct after sequencing, as shown in figure 2 a.
[0089] The recombinant plasmid obtained above was transfected into a human kidney epithelial cell line (293T), and the human kidney epithelial cell line (293T) transformed with an empty plasmid pcDNA3.1(+) was used as a control. The blank group was only transfected with pcDNA3.1(+) plasmid, and the miPEP155 group was transfected with miPEP-histag plasmid. Immunofluorescence microscopy was used to observe the expression of miPEP-histag protein. The result is as figure 2 B, miPEP-histag protein is mainly expressed in the envelope and cytoplasm of 293T cells.
Embodiment 3
[0090] Example 3, miPEP155 inhibits Th17 cell differentiation
[0091] The miPEP155 obtained by the solid-phase peptide synthesis method in Example 1 was tested for its effect on Th17 cell differentiation.
[0092] Obtain mouse spleen cells and isolate mice using immunomagnetic beads CD4+ T cells were cultured in 1640 medium at 37°C, and anti-CD3 (5 μg / ml), anti-CD28 (2 μg / ml), TFG-β (10 ng / ml), IL -23 (20 ng / ml), anti-IL-4 (5 μg / ml), anti-IFN-γ (10 μg / ml) were cultured for 3 days to obtain Th17.
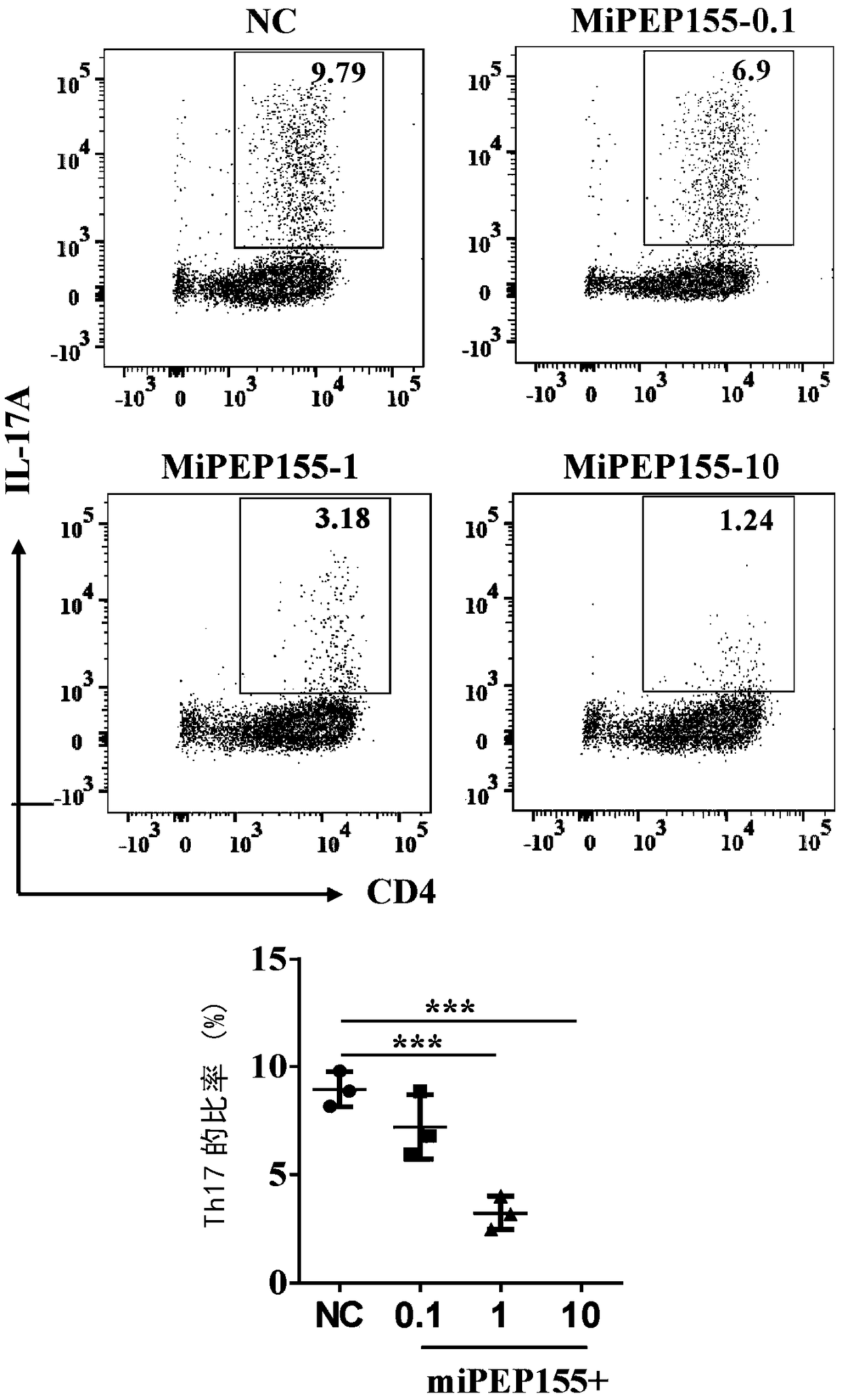
[0093] Th17 cells were cultured in 1640 medium at 37°C, divided into several culture groups, and miPEP155 at concentrations of 0 μM, 0.1 μM, 1 μM and 10 μM were added, and the effect of miPEP155 on Th17 differentiation was observed by flow cytometry.
[0094] The result is as image 3 , miPEP155 can significantly inhibit the differentiation of Th17 induced in vitro.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com