Percutaneous absorption composition and use thereof in preparation of percutaneous absorption preparation
A composition and preparation technology, which is applied in the field of transdermal drug delivery preparations, can solve the problems of limited permeation enhancement effect, achieve the effects of reducing toxic side effects and skin irritation, improving stability and prolonging release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Pre-treatment of pigskin: take fresh pigskin, remove the subcutaneous fat layer, shave the pig hair to a length no longer than 2mm, and use it immediately or freeze it at -20°C to -80°C for later use.
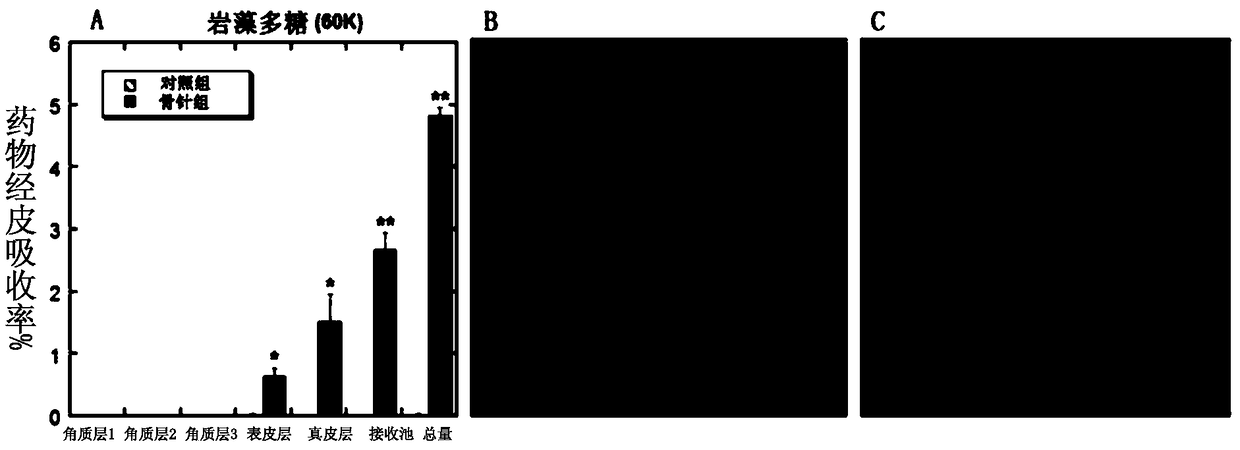
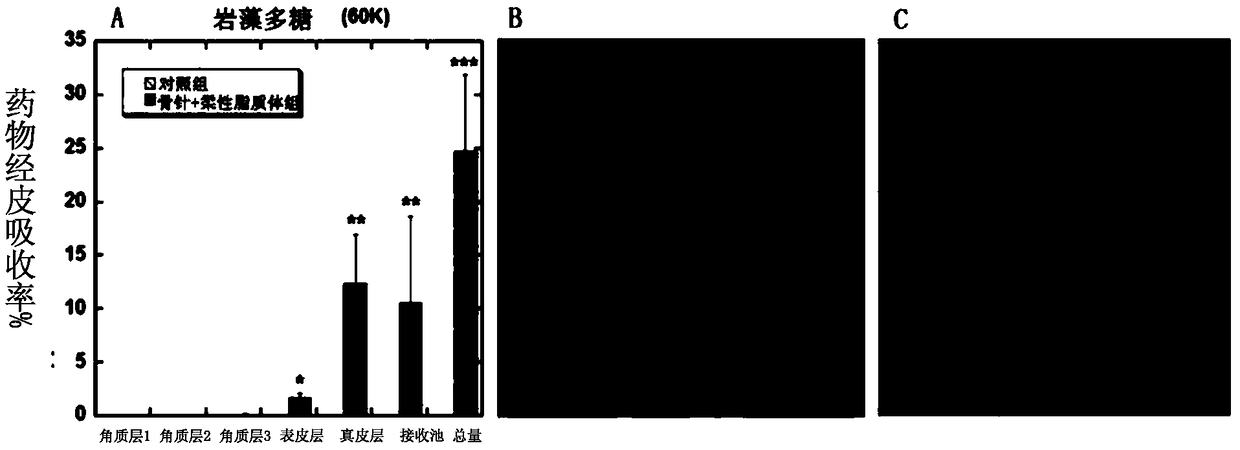
[0052] (2) Preparation of flexible nanoliposomes: phospholipid 90G (100mg / ml), surfactant polyoxyethylene (20) oleyl ether (100mg / ml) and methanol / chloroform=1:1 were added to the In a round flask, it was evaporated to dryness by a rotary evaporator to form a uniform film on the bottom surface of the round flask. Then add 1 to 5 ml of ANTS-Fucoidan (fucoidan) drug solution, and perform hydration by shaking / ultrasound and other steps. Use the imported special extrusion device for liposomes, squeeze 21 times, and finally transfer to a new EP tube to obtain ANTS-Fucoidan flexible nano-liposome solution of 1-100 mg / ml, ready for use.
[0053] (3) Preparation of sponge spicule solution: add a certain amount of PBS phosphate buffer saline, deionized water, normal saline o...
Embodiment 2
[0059] (1) Pretreatment of pigskin: with embodiment 1.
[0060] (2) Preparation of ordinary nanoliposomes: add phospholipid 90G (100mg / ml) and methanol / chloroform=1:1 into a round flask in a certain ratio, evaporate to dryness by a rotary evaporator, and make it in a round shape Form a uniform film on the bottom surface of the flask; then add 1 to 5ml of FITC-Hyaluronic acid (hyaluronic acid) drug solution, hydrate through oscillation / ultrasound and other steps, use imported liposome special extrusion device, and squeeze 21 times , and finally transferred to a new EP tube to obtain a 1-100 mg / ml FITC-Hyaluronicacid common nanoliposome solution for use.
[0061] (3) Preparation of flexible nanoliposomes: similar to Example 1.
[0062] (4) Preparation of sponge spicule solution: same as Example 1.
[0063] (5) In vitro skin penetration test: similar to Example 1. Add 150 μL of FITC-Hyaluronic acid (average molecular weight: 250KDa) solution containing 1 to 100 mg / ml, or FITC-...
Embodiment 3
[0071] (1) Pretreatment of pigskin: with embodiment 1.
[0072] (2) Preparation of solid lipid nanoparticles: prepared with reference to conventional techniques in the art.
[0073] (3) Preparation of sponge spicule solution: same as in Example 1.
[0074] (4) In vitro transdermal test: carried out with reference to Example 1. The results showed that the spongy spicules combined with solid lipid nanoparticles had obvious penetration-promoting effect compared with other groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com