Method for preparing pymetrozine
A technology of pymetrozine and pyridine, which is applied in the field of preparation of pymetrozine, can solve the problems of high toxicity of phosgene or solid phosgene, difficulty in monitoring the reaction, and low atom economy, and achieve easy recycling and low production cost , The effect of high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
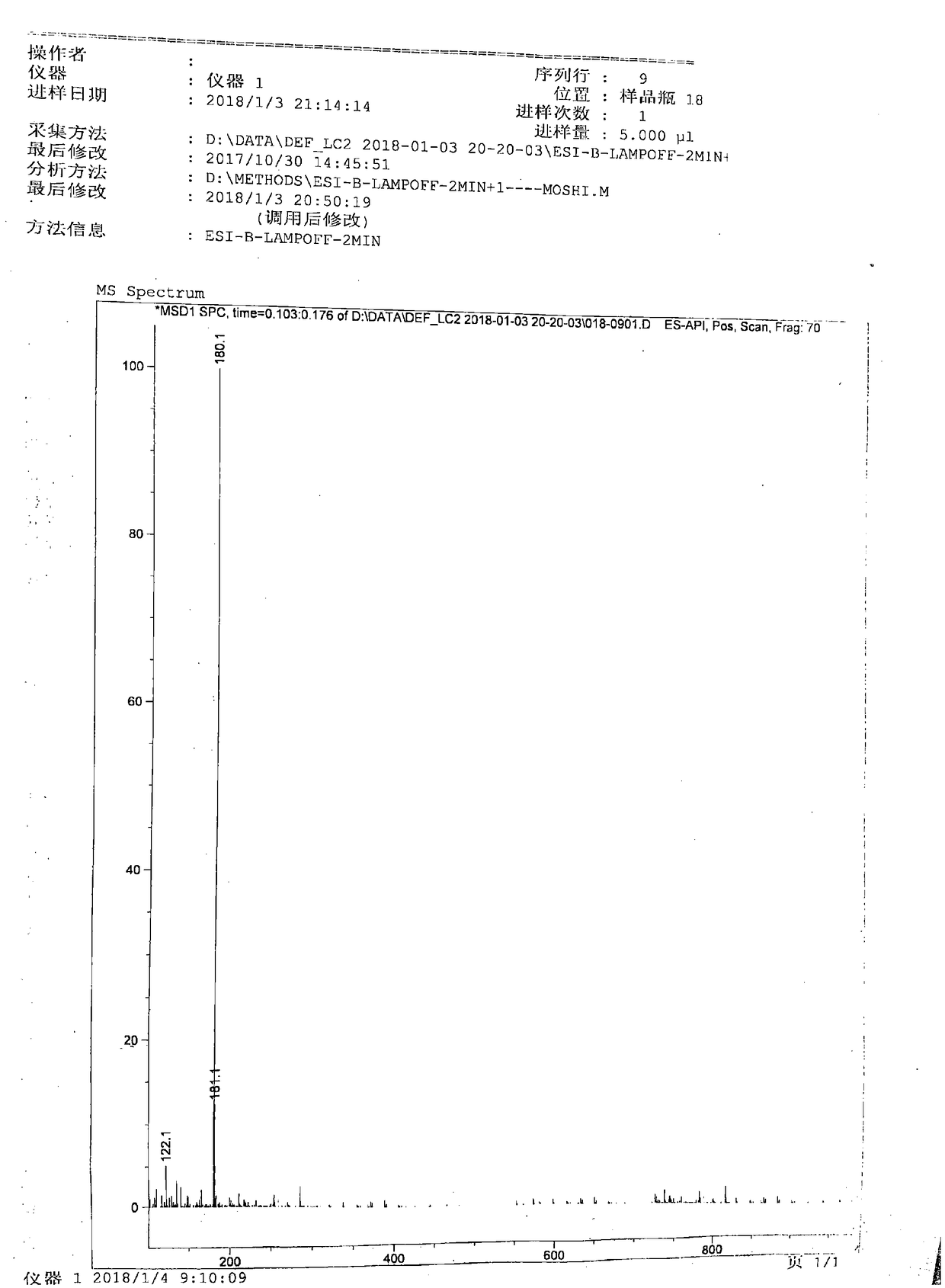
[0052] 1) In a 2000mL reaction flask, install a stirrer, a thermometer, a constant pressure dropping funnel and a condenser. Add 540 mL of dimethyl carbonate at room temperature, start stirring, add 480 mL of 80% hydrazine hydrate, react at 70°C for 4 hours, stop the reaction, recover unreacted dimethyl carbonate, methanol and water by distillation under reduced pressure, and add 80% hydrazine hydrate 960mL, continued to react at 80°C for 4h, cooled to room temperature, stirred, a large amount of white solid precipitated, filtered with suction to obtain a white solid, rinsed with a small amount of methanol (100mL×3), dried to obtain 450g white solid, yield 83 %, melting point 153-155°C.
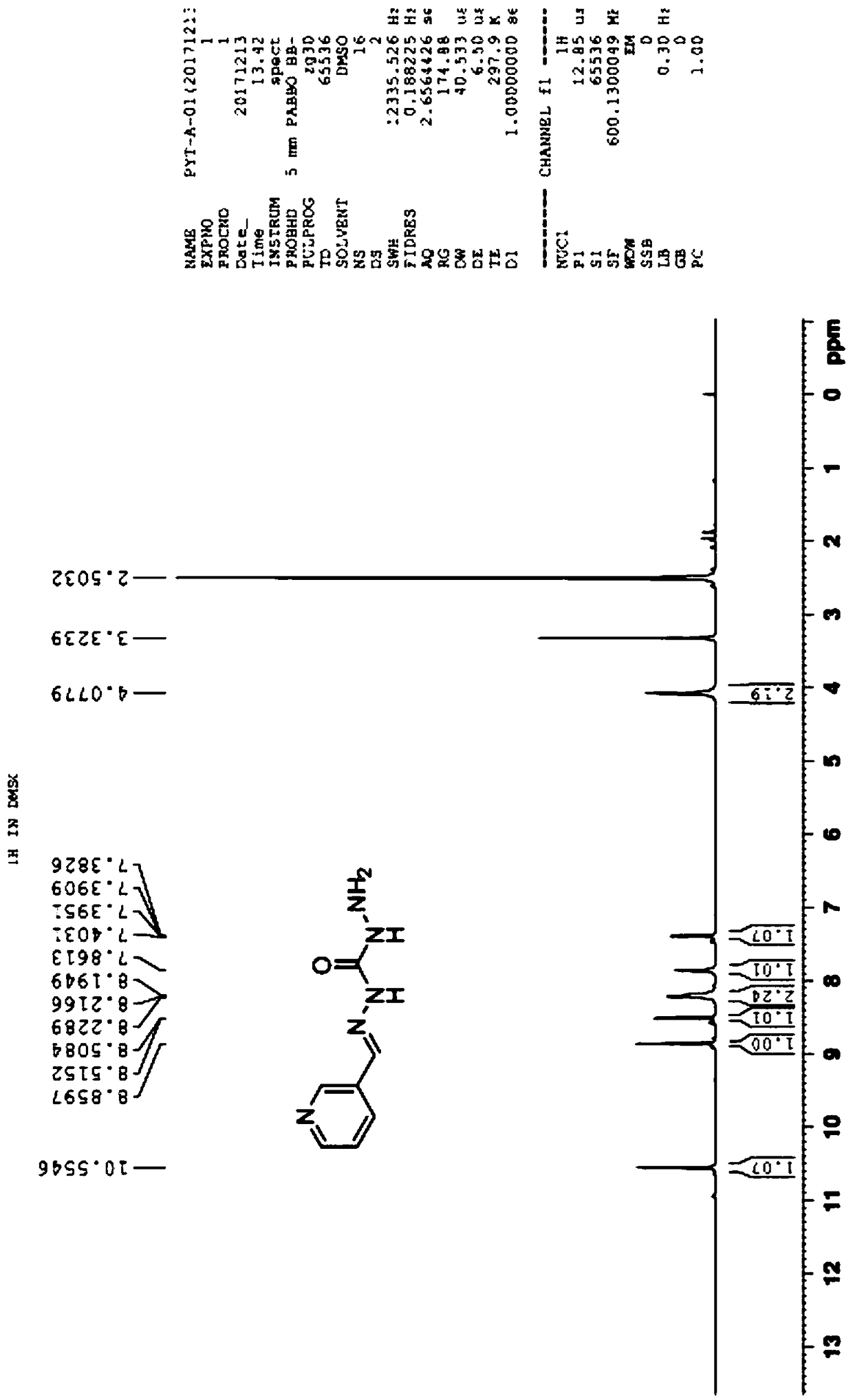
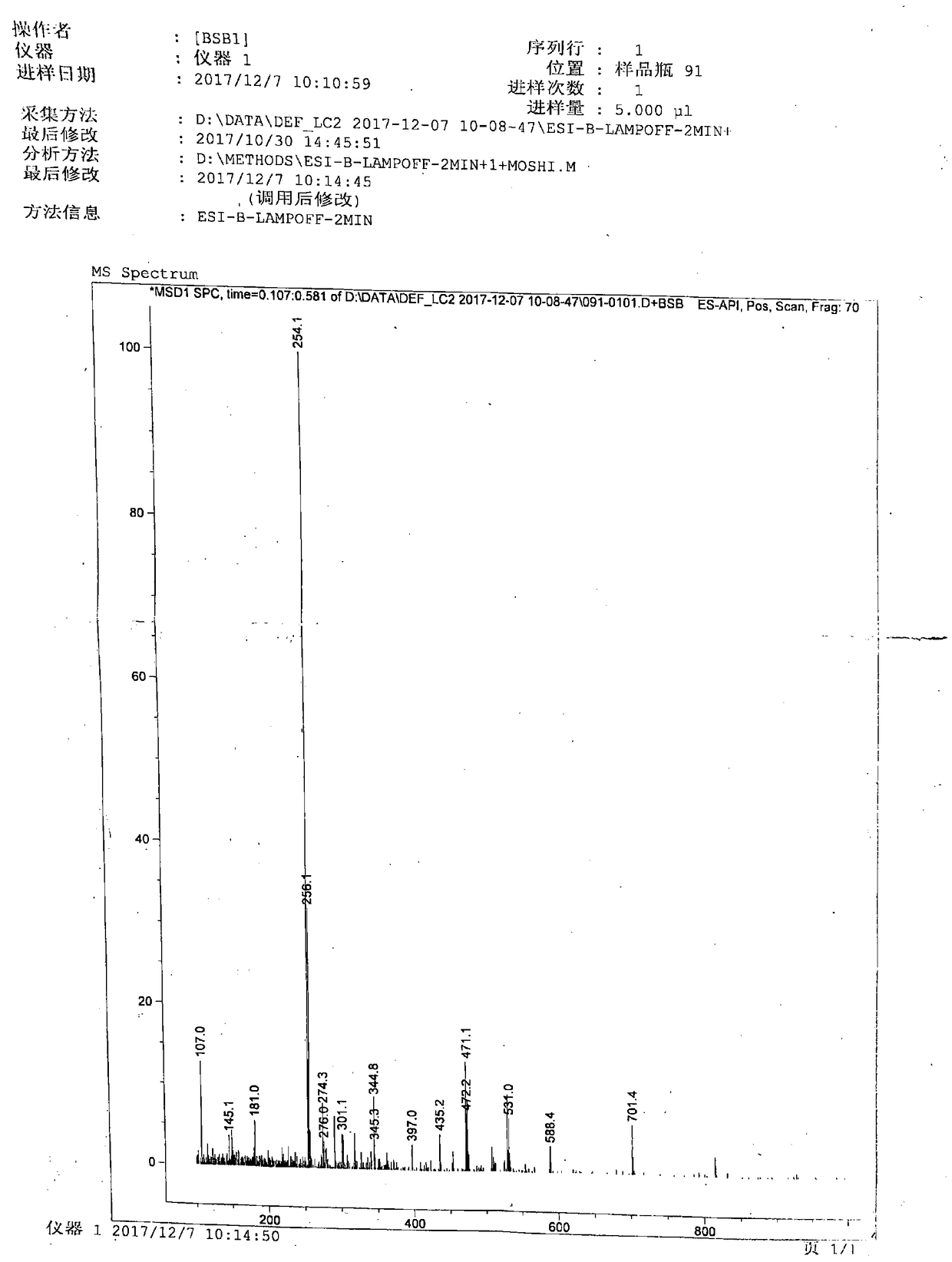
[0053]2) In a 2000mL reaction bottle, install a stirrer, a thermometer, and a constant pressure dropping funnel, add 500mL of pure water at room temperature, start stirring, slowly add 450g of carbazide, stir to dissolve, cool down to 0-5°C, and add dropwise Nicotinic aldehyde 540g, temperat...
Embodiment 2
[0057] 1) In a 2000mL reaction flask, install a stirrer, a thermometer, a constant pressure dropping funnel and a condenser. Add 540 mL of dimethyl carbonate at room temperature, start stirring, add 1000 mL of hydrazine-containing methanol solution (hydrazine accounts for 60% of the solution), stop the reaction after reflux reaction at 70°C for 4 hours, and recover unreacted dicarbonate by distillation under reduced pressure. Methyl ester, methanol, add 650mL of methanol solution containing hydrazine to the residue, continue to reflux vigorously at 80°C for 4h, cool to room temperature, stir, a large amount of white solid precipitates, filter with suction to obtain white solid with a small amount of methanol (100mL× 3) Rinse and dry to obtain 460 g of white solid with a yield of 85% and a melting point of 152-154°C.
[0058] 2) In a 2000mL reaction bottle, install a stirrer, a thermometer, and a constant pressure dropping funnel, add 500mL of ethanol at room temperature, start...
Embodiment 3
[0062] 1) In a 2000mL reaction flask, install a stirrer, a thermometer, a constant pressure dropping funnel and a condenser. Add 540 mL of dimethyl carbonate at room temperature, start stirring, add 480 mL of ethanol solution of hydrazine (hydrazine accounts for 80% of the solution by volume), stop the reaction after reacting at 70°C for 4 hours, and recover unreacted dimethyl carbonate by distillation under reduced pressure , methanol and ethanol, add 540mL of ethanol solution of hydrazine to the residue, continue to react at 80°C for 4h, cool to room temperature, stir, a large amount of white solid precipitates, filter with suction, and rinse the white solid with a small amount of methanol (100mL×3) After washing and drying, 455 g of white solid was obtained, the yield was 84%, and the melting point was 154-156°C.
[0063] 2) In a 2000mL reaction flask, install a stirrer, a thermometer, and a constant pressure dropping funnel, add 500mL of isopropanol at room temperature, st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com