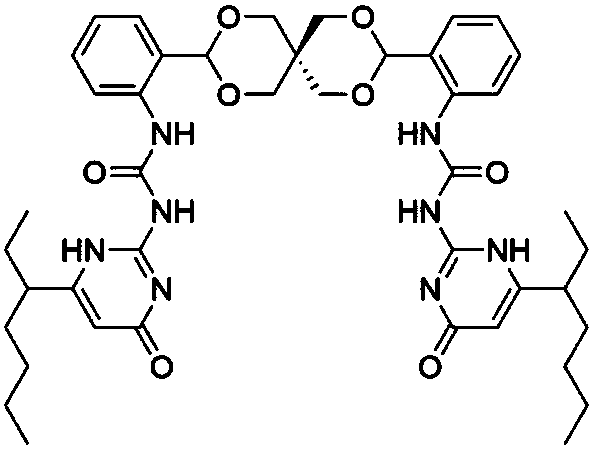
Oxa-spiro bridged UPy compound and synthesis method thereof
A technology of ureido pyrimidinone and bis-ureido pyrimidinone is applied in the field of synthesis technology of oxa spiro ring bridged ureido pyrimidinone compound, can solve the problems of lack of dynamic reversibility, poor degradability and the like, and achieves good application prospects , mild reaction conditions, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
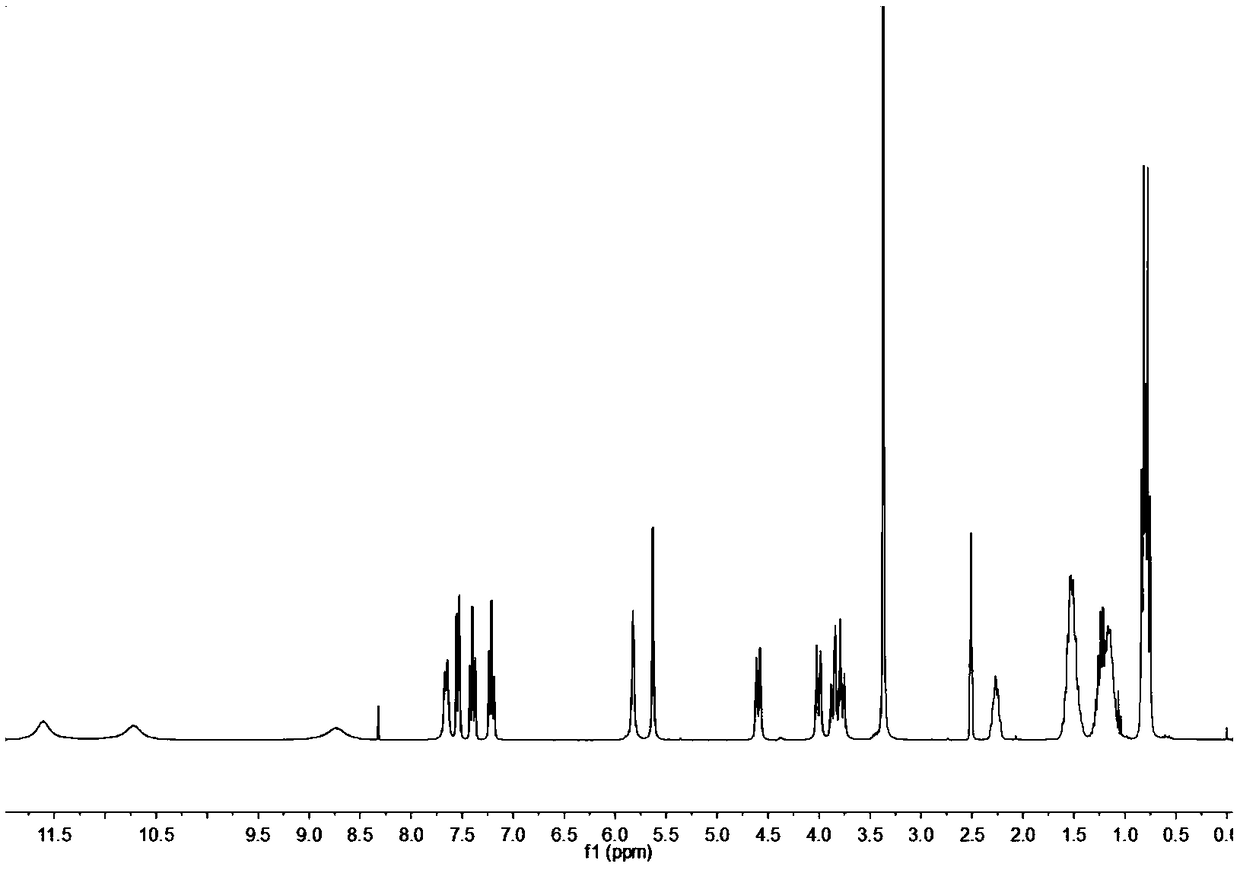
Embodiment 1
[0031] Synthesis of step 1, oxaspiro dinitro compound (compound A):
[0032] Add 5.00 g of o-nitrobenzaldehyde, 2.25 g of pentaerythritol and 0.30 g of catalyst p-toluenesulfonic acid into a 250 mL three-necked flask, then add 40 mL of toluene as a solvent, and set up a water separator to remove water in the reaction. Maintain 140°C, stir the reaction, reflux for 3h, and stop heating. Continue to reflux until the temperature of the reaction system drops to room temperature and stop. Rotary evaporation, remove toluene, with saturated NaHCO 3 The solution was washed (50mL×2), and then washed with distilled water (50mL×2), and the crude product was obtained by suction filtration. Finally, with CH 3 OH / CH 2 Cl 2 After recrystallization, suction filtration and drying at 50° C. for 3 h under vacuum, the product is a brown powdery solid with a yield of 98.80%.
[0033] Step 2, the synthesis of oxaspiro diamino compound (compound B):
[0034] Add 6.00 g of compound A and 0.40 g...
Embodiment 2~6
[0041] The synthesis of Compound A, Compound B and Compound C is the same as in Example 1, and the synthesis of Compound D is synthesized with reference to step 4 of Example 1, only changing Compound B: The molar ratio of Compound C is 1:2.0, 1:2.1, 1: 2.3, 1:2.4, 1:2.5, the target product is obtained.
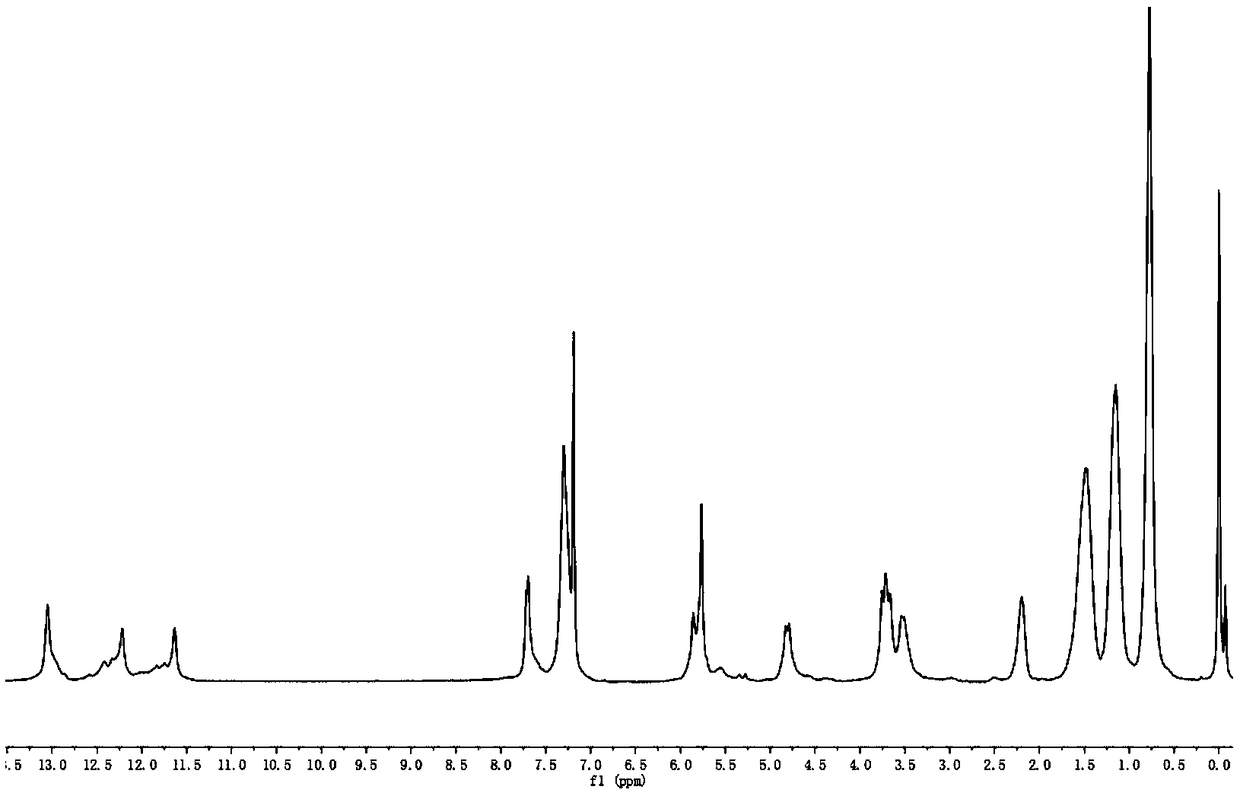
Embodiment 7
[0043] Step 1, the synthesis of compound A was synthesized with reference to Example 1.
[0044] Step 2, the synthesis of compound B was synthesized with reference to Example 1.
[0045] Synthesis of step 3, activated ureidopyrimidinone compound (compound C):
[0046] Add 0.79g of 6-isoheptylisopyrimidine and 1.14g of N,N'-carbonyldiimidazole (CDI) into a 50mL three-necked flask, and exhaust the oxygen and moisture. Under nitrogen protection, add CHCl after washing with water for 15 times and drying 3 20mL, stirred at room temperature for 6h, and stopped the reaction. Wash with water (30 mL×2) and saturated brine (30 mL×2) successively, collect the organic phase, dry over anhydrous magnesium sulfate, and filter.
[0047] Step 4, the synthesis of compound D was synthesized with reference to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com