Bacillus subtilis glycosyltransferase and application thereof
A technology of glycosyltransferase and glycosyl, applied in the field of glycosyltransferase, can solve the problems of many by-products, high cost and low yield of chemical synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1. Cloning of Glycosyltransferase Gene BsUGT1
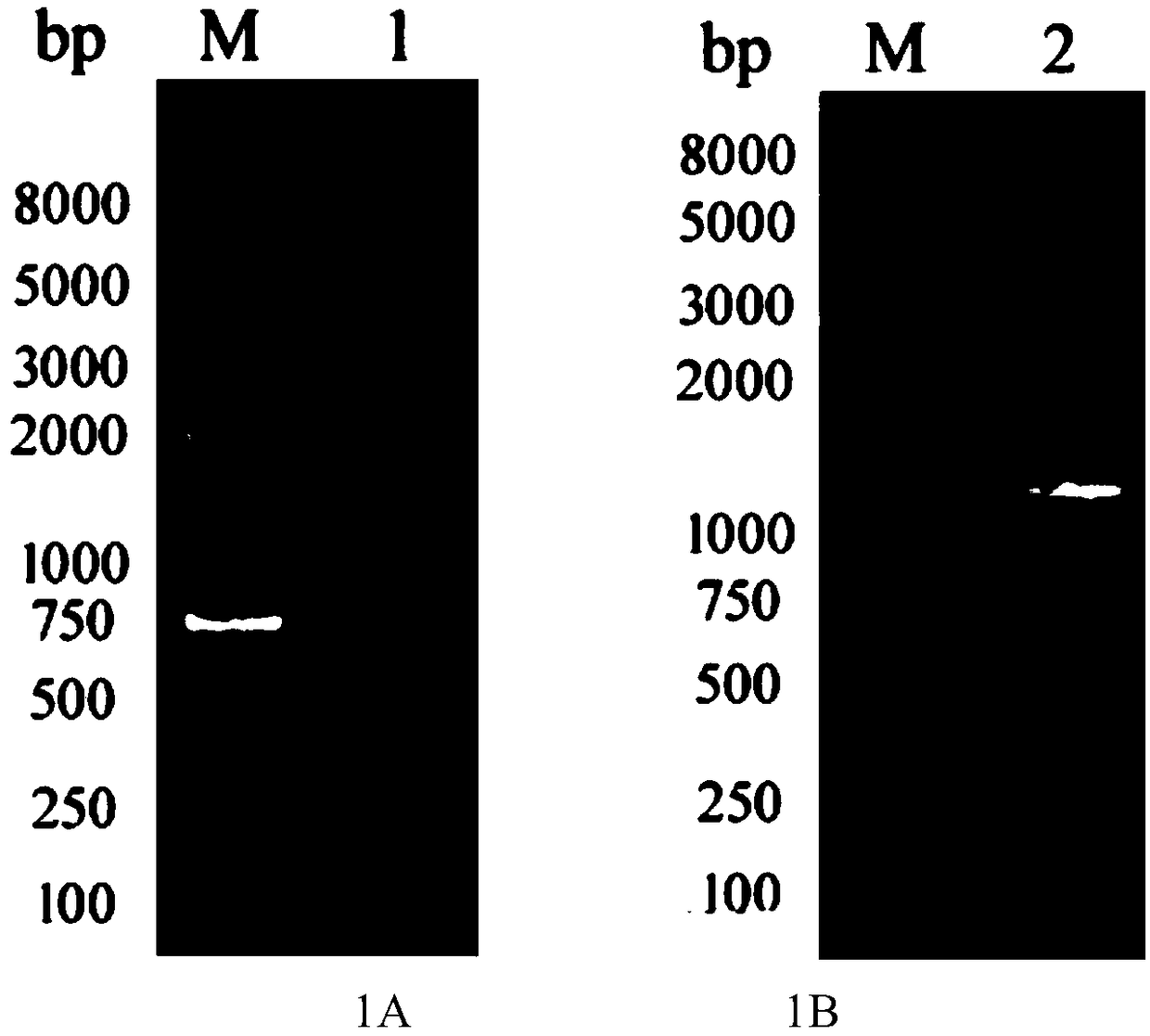
[0096] Bacillus subtilis (Bacillus subtilis CTCC63501) genomic DNA was extracted using a bacterial genomic DNA extraction kit ( figure 1 A). Using Bacillus subtilis genomic DNA as a template, PCR amplification was performed using primers BsUGT1-F (SEQ ID NO: 3) and BsUGT1-R (SEQ ID NO: 4) to obtain the glycosyltransferase gene BsUGT1 (SEQ ID NO: 2) . Wherein, the sequences of the primers BsUGT1-F (SEQ ID NO: 3) and BsUGT1-R (SEQ ID NO: 4) used are listed in Table 1. PCR products were detected in 1.0% agarose gel electrophoresis ( figure 1 B).
[0097] Table 1 Primer Sequence
[0098]
[0099] PCR reaction system and reaction conditions:
[0100] PCR reaction system (50μL)
[0101]
[0102] PCR reaction conditions
[0103] 98℃, 2min
[0104] 98℃, 15s; 55-60℃, 30s; 72℃, 1kb / 15-30s; 30 cycles
[0105] 72℃, 10min
[0106] 4°C, ∞
[0107] After the PCR reaction, 4 μL of the PCR product was connected to...
Embodiment 2
[0109] Example 2. Prokaryotic expression of glycosyltransferase BsUGT1
[0110] According to the sequence information obtained from the sequencing of plasmid pEASY-Blunt-BsUGT1, primers BsUGT1-F1 (SEQ ID NO: 5) and BsUGT1-R1 (SEQ ID NO: 6) were designed to amplify the same sequence as the prokaryotic expression plasmid pET-32a(+). The PCR fragment BsUGT1 of the source arm was simultaneously introduced with restriction enzyme cutting sites BamH I and Sal I, and the primer sequences are shown in Table 2.
[0111] Table 2 Primer Sequence
[0112]
[0113] PCR reaction system and reaction conditions:
[0114] PCR reaction system (50μL)
[0115]
[0116] PCR reaction conditions
[0117] 98℃, 2min
[0118] 98℃, 15s; 55-60℃, 30s; 72℃, 1kb / 15-30s; 30 cycles
[0119] 72℃, 10min
[0120] 4°C, ∞
[0121] The expression vector pET-32a(+) was double digested with restriction endonucleases BamH I and Sal I. The PCR fragment and the double-enzyme-digested vector were gel-cut an...
Embodiment 3
[0126] Example 3. Detection of recombinant BsUGT1 catalyzed DM glycosylation activity
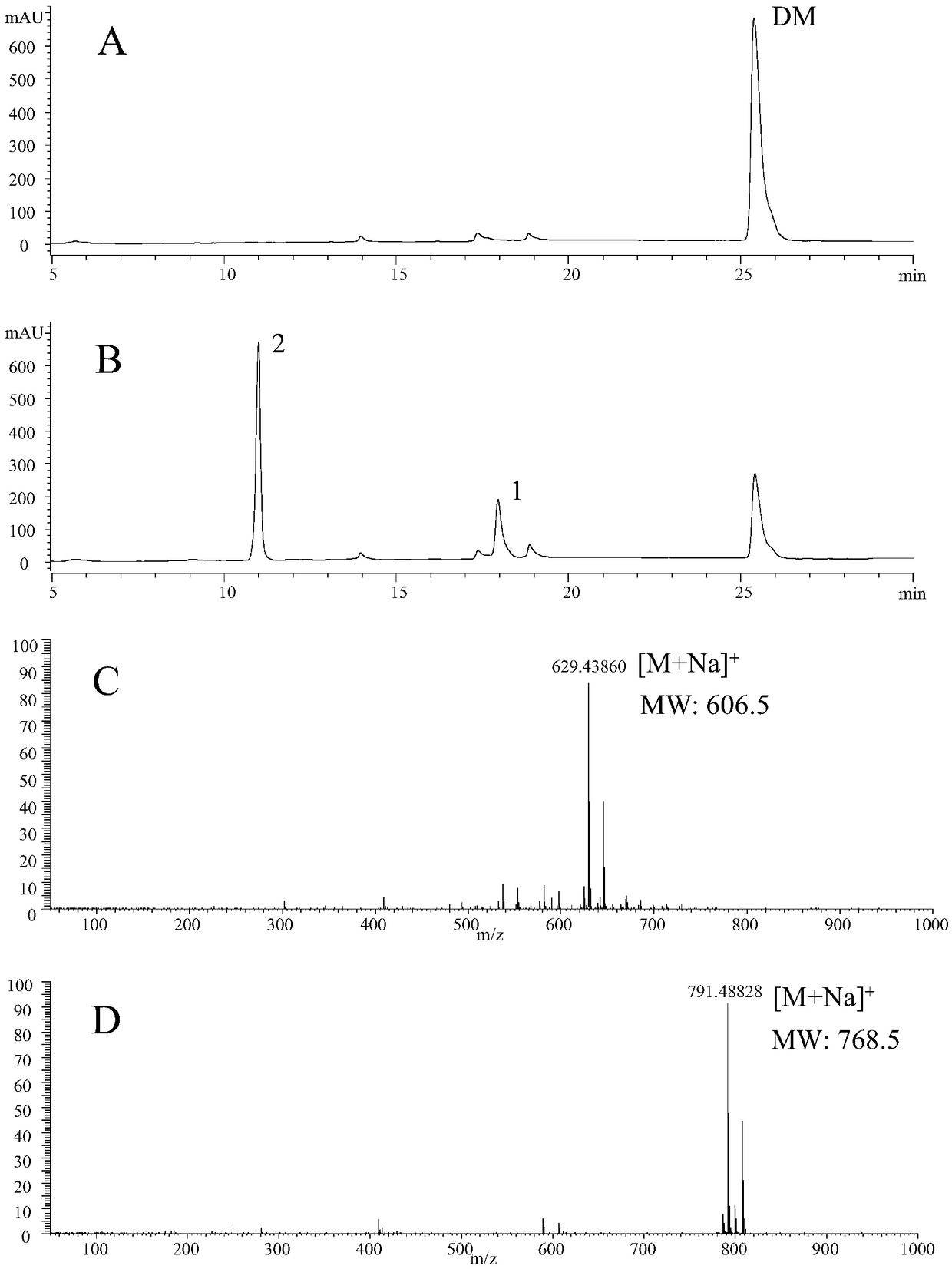
[0127] Using Transetta-32a cells as negative control group, Transetta-32a cells and Transetta-BsUGT1 cells were sonicated. The crushed supernatant was used as the crude enzyme solution, UDP-glucose was used as the glycosyl donor, and DM was used as the substrate to carry out the enzymatic reaction in vitro.
[0128] Glycosyltransferase catalytic activity identification system: 100μL, 20mM Tris-HCl (pH 8.0)
[0129] Crude enzyme solution: 88μL
[0130] 50mM substrate: 2μL
[0131] 50mM UDPG: 10μL
[0132] The reaction system was mixed well, and left to react at 37°C for 24 hours, then 200 μL of ice methanol was added to terminate the reaction, mixed well, centrifuged at 12,000 rpm for 10 minutes, the supernatant was passed through a 0.45 μm filter membrane, and the glycosylation product of DM catalyzed by BsUGT1 was detected by HPLC.
[0133] HPLC detection conditions: Cosmosil C18 rever...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com