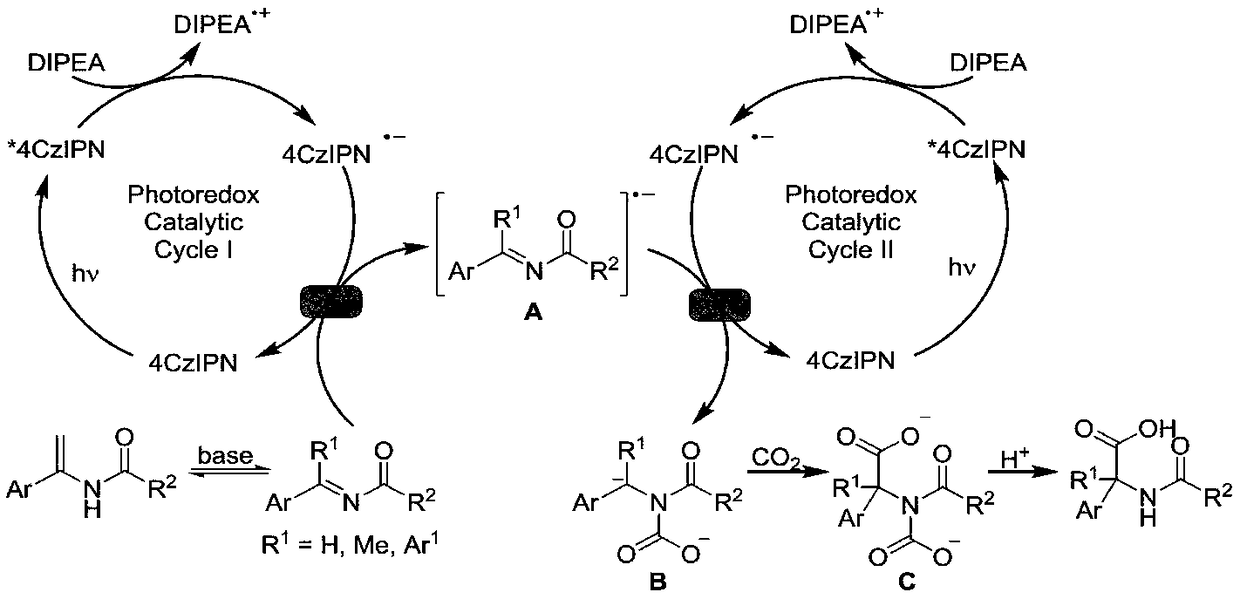
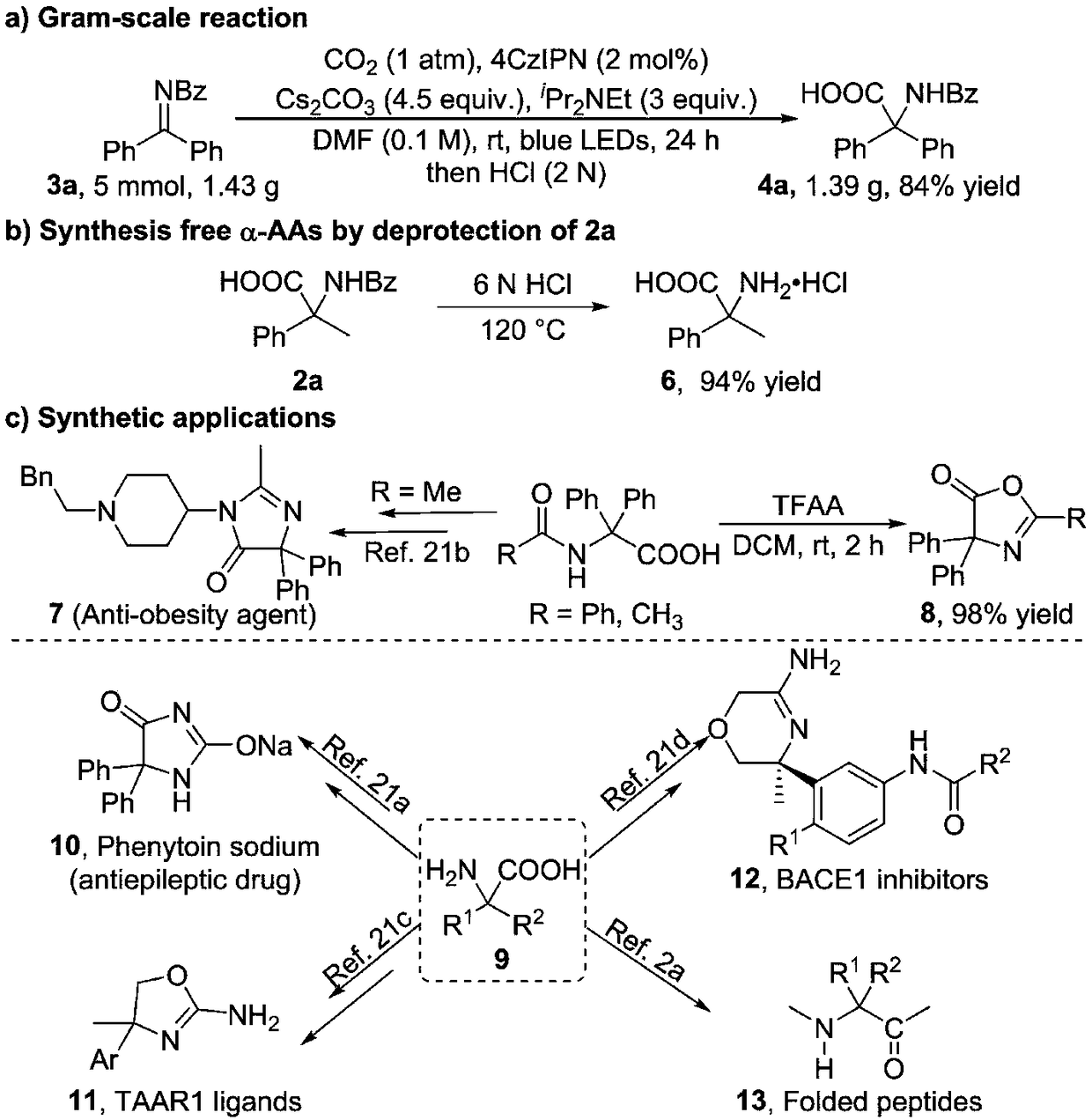
Method for synthesizing alpha-quaternary carbon amino acid
A synthetic method and amino acid technology, applied in the field of synthesis of α-quaternary carbon amino acids, can solve difficult problems such as the synthesis of α-quaternary carbon amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] To a dry Schlenk tube (10 mL) containing magnetons was added the reaction substrate (0.2 mmol), 4CzIPN (4 mg, 0.004 mmol, 2 mol%). The Schlenk tube was then transferred into the glove box, where it was loaded with Cs 2 CO 3 (294mg, 0.9mmol, 4.5 times equivalent). Remove the Schlenk tube from the glove box and connect to a CO 2 On the double row pipe of the steel cylinder, pump and charge CO on the double row pipe 2 At least 3 times, drain the N in the tube 2 , so that the tube is filled with CO 2 gas. Then in CO 2 Add DMF (2mL) and i PR 2 NEt (100 μL, 3 equiv.). Finally, the reaction solution was placed 2-4 cm away from the 30W blue LED, and stirred at room temperature (25° C.) for 4 hours. The mixture was then quenched with 1 mL of water, 2.5 mL of ethyl acetate and 2 mL of 2N hydrochloric acid, then directly concentrated and spin-dried. The residue was purified by flash column chromatography (first with petroleum ether / ethyl acetate 5 / 1 (v / v) and 0.2-0.3% g...
Embodiment 2
[0070] To a dry Schlenk tube (10 mL) containing magnetons was added the reaction substrate (0.2 mmol), 4CzIPN (2 mg, 0.002 mmol, 1 mol%). The Schlenk tube was then transferred into a glove box, which contained K 2 CO 3 (139 mg, 1.0 mmol, 5 equivalents). Remove the Schlenk tube from the glove box and connect to a CO 2 On the double row pipe of the steel cylinder, pump and charge CO on the double row pipe 2 At least 3 times, drain the N in the tube 2 , so that the tube is filled with CO 2 gas. Then in CO 2 Add DMF (2mL) and i PR 2 NEt (67 μL, 2 equiv). Finally, the solution was placed 2-4cm away from the 30W blue LED, and stirred at room temperature (25°C) for 4 hours. The mixture was then quenched with 1 mL of water, 2.5 mL of ethyl acetate and 2 mL of 2N hydrochloric acid, then directly concentrated and spin-dried. The residue was purified by flash column chromatography (first with petroleum ether / ethyl acetate 5 / 1 (v / v) and 0.2-0.3% glacial acetic acid, then with p...
Embodiment 3
[0072] To a dry Schlenk tube (10 mL) containing magnetons was added the reaction substrate (0.2 mmol), 4CzIPN (10 mg, 0.01 mmol, 5 mol%). The Schlenk tube was then transferred into a glove box, where it was filled with Na 2 CO 3 (106mg, 1.0mmol, 5 times equivalent). Remove the Schlenk tube from the glove box and connect to a CO 2 On the double row pipe of the steel cylinder, pump and charge CO on the double row pipe 2 At least 3 times, drain the N in the tube 2 , so that the tube is filled with CO 2 gas. Then in CO 2 Add DMF (2mL) and i PR 2 NEt (167 μL, 5 equivalents). Finally, the solution was placed 2-4 cm away from the 30W blue LED, and stirred at room temperature (25° C.) for 4 hours. The mixture was then quenched with 1 mL of water, 2.5 mL of ethyl acetate and 2 mL of 2N hydrochloric acid, then directly concentrated and spin-dried. The residue was purified by flash column chromatography (first with petroleum ether / ethyl acetate 5 / 1 (v / v) and 0.2-0.3% glacial a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com