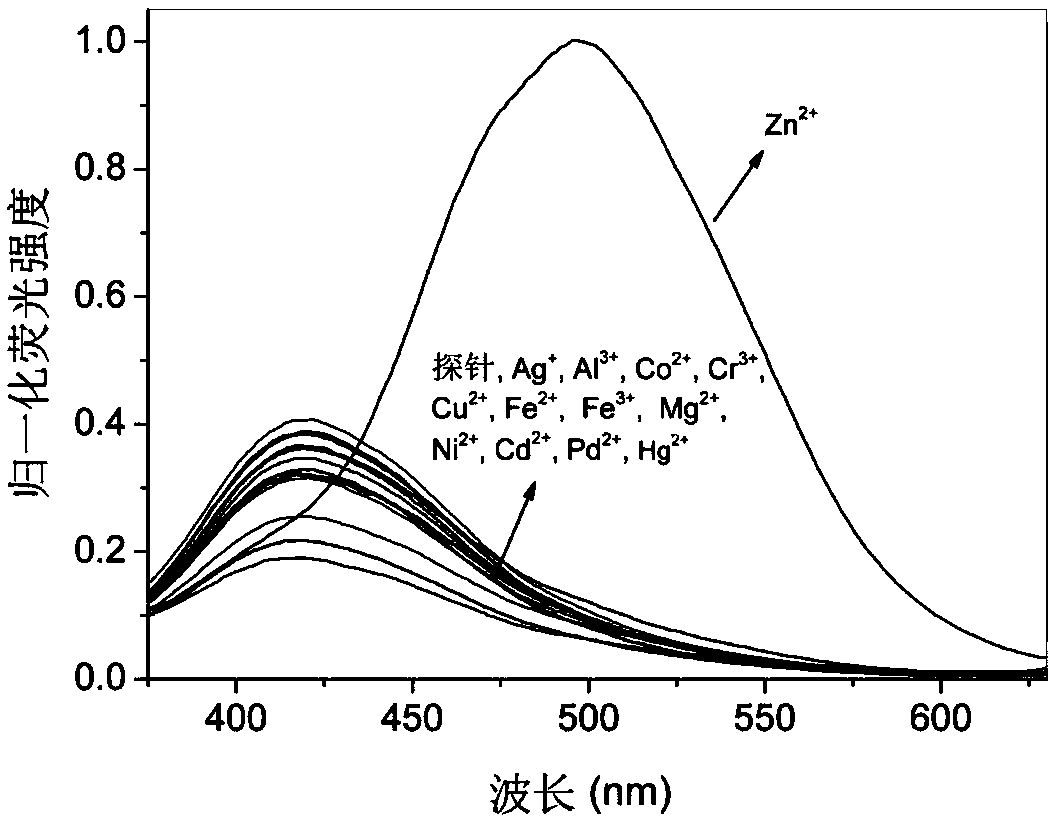
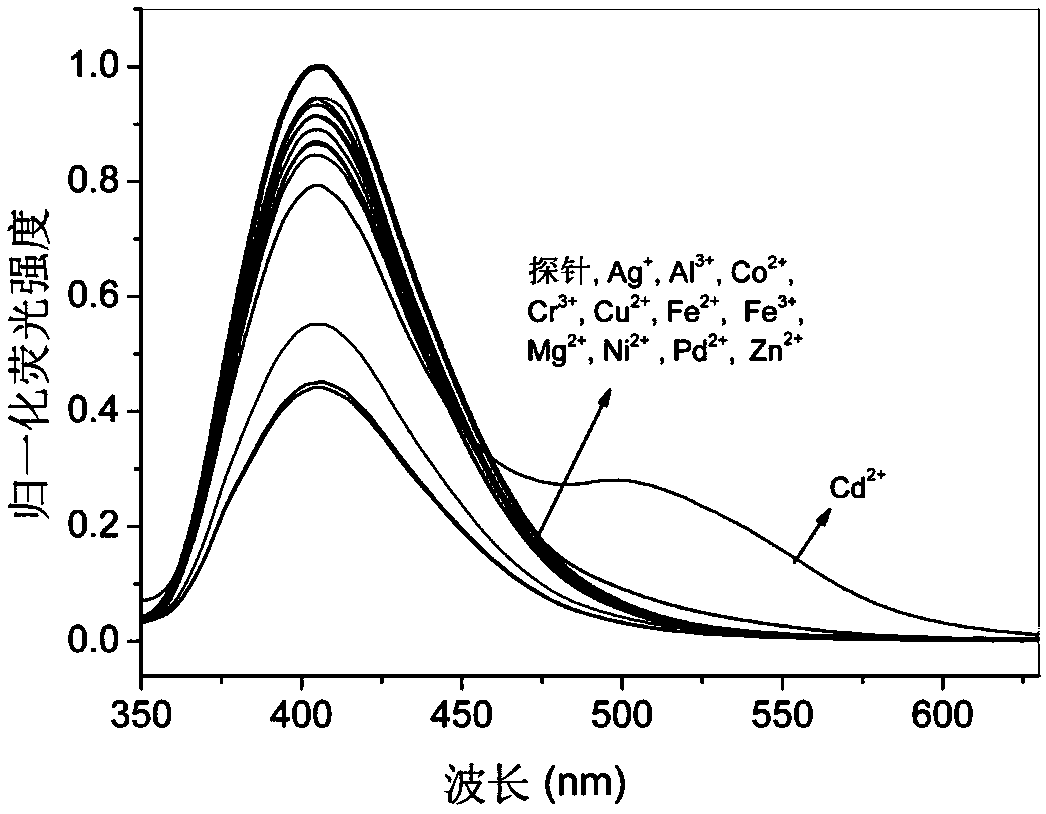
8-aminoquinoline amide derivative, preparation method, application and fluorescence analysis method thereof
An aminoquinoline amide, aminoquinoline technology, applied in derivatives, preparation methods, fluorescence analysis based on derivatives, application fields, can solve problems such as fluorescence detection signal interference, ion detection interference, etc., to reduce fluorescence signal measurement. Effects of interference, improved selectivity, excellent spectral performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation method of 8-aminoquinoline amide derivative of the present invention comprises the following steps:
[0039] 1a) Weigh N-bromoacetyl-8-aminoquinoline, propynylamine and acetonitrile, then dissolve N-bromoacetyl-8-aminoquinoline and propynylamine in acetonitrile, and then add potassium carbonate, A reaction mixture was obtained, wherein the ratio of N-bromoacetyl-8-aminoquinoline, propargylamine, potassium carbonate and acetonitrile was 100mg: 20mg: 100mg: 5mL;
[0040] 2a) The reaction mixture obtained in step 1a) was stirred and reacted at room temperature, then filtered to remove the solvent, and then eluted by column chromatography to obtain 8-aminoquinoline amide derivatives.
[0041] The time of stirring the reaction at room temperature in step 2a) is 36h;
[0042] In the specific process of column chromatography elution in step 2a), a mixture of petroleum ether and ethyl acetate with a boiling point of 60-90° C. is used as an eluent for elution, w...
Embodiment 2
[0044] The preparation method of 8-aminoquinoline amide derivative of the present invention comprises the following steps:
[0045] 1a) Weigh N-bromoacetyl-8-aminoquinoline, propynylamine and acetonitrile, then dissolve N-bromoacetyl-8-aminoquinoline and propynylamine in acetonitrile, and then add potassium carbonate, A reaction mixture was obtained, wherein the ratio of N-bromoacetyl-8-aminoquinoline, propargylamine, potassium carbonate and acetonitrile was 100mg: 70mg: 50mg: 10mL;
[0046] 2a) The reaction mixture obtained in step 1a) was stirred and reacted at room temperature, then filtered to remove the solvent, and then eluted by column chromatography to obtain 8-aminoquinoline amide derivatives.
[0047] The time of stirring the reaction at room temperature in step 2a) is 12h;
[0048] In the specific process of column chromatography elution in step 2a), a mixture of petroleum ether and ethyl acetate with a boiling point of 60-90° C. is used as an eluent for elution, w...
Embodiment 3
[0050] The preparation method of 8-aminoquinoline amide derivative of the present invention comprises the following steps:
[0051] 1a) Weigh N-bromoacetyl-8-aminoquinoline, propynylamine and acetonitrile, then dissolve N-bromoacetyl-8-aminoquinoline and propynylamine in acetonitrile, and then add potassium carbonate, A reaction mixture was obtained, wherein the ratio of N-bromoacetyl-8-aminoquinoline, propargylamine, potassium carbonate and acetonitrile was 100mg: 50mg: 80mg: 8mL;
[0052] 2a) The reaction mixture obtained in step 1a) was stirred and reacted at room temperature, then filtered to remove the solvent, and then eluted by column chromatography to obtain 8-aminoquinoline amide derivatives.
[0053] The time of stirring the reaction at room temperature in step 2a) is 25h;
[0054] In the specific process of column chromatography elution in step 2a), a mixture of petroleum ether and ethyl acetate with a boiling point of 60-90° C. is used as an eluent for elution, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com