Preparation method of 2-benzofuranone compound
A technology of benzofuranone and compounds, which is applied in the field of preparation of 3-benzofuranone compounds, can solve the problems of cumbersome operation and long reaction time, and achieve the effects of simple operation, short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
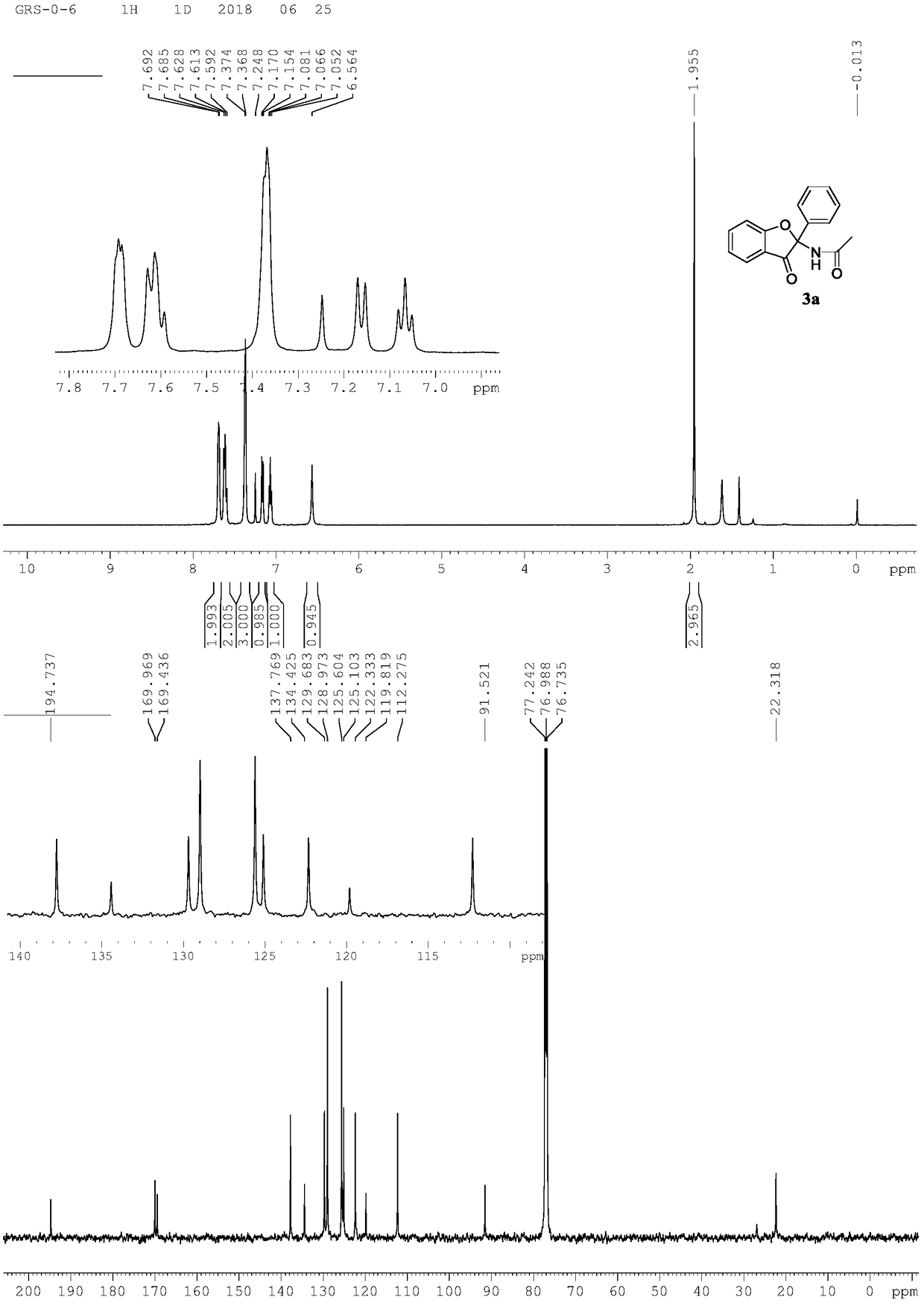
[0023] Preparation of 3-benzofuranone compound 3a
[0024]
[0025] Add N-phenoxyacetamide (0.2mmol, 30mg), phenylpropionic acid (0.2mmol, 29mg), 5% rhodium catalyst, cobalt acetate hydrate, sodium pivaloate hydrate, methanol 1.0mL to 15mL thick In the wall pressure tube, stir for 12 hours at room temperature with an open opening. After the reaction, it was separated by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=4 / 1) to obtain N-(3-oxo-2-phenyl-2,3-dihydrobenzo Furan-2-yl)acetamide 3a (0.184mmol, 49mg), isolated yield 92%.
[0026] Spectral analysis data 3a:
[0027] 1 H NMR(500MHz, CDCl 3 ):δ7.69-7.68(m,2H),7.63-7.59(m,2H),7.37-7.36(m,2H),7.16(d,J=8.15Hz,1H), 7.07(t,J=7.35 Hz, 1H), 6.56 (s, 1H), 1.95 (s, 1H). 13 C NMR(125MHz, CDCl 3 ): δ194.7,170.0,169.4,137.8,134.4,129.7,128.9,125.6,125.1,122.3,119.8,112.2,91.5,22.3.HRMS(ESI-TOF,[M+Na] + ):calcd for C 16 H 13 NO 3 Na, 290.0793, found 290.0796.
Embodiment 2
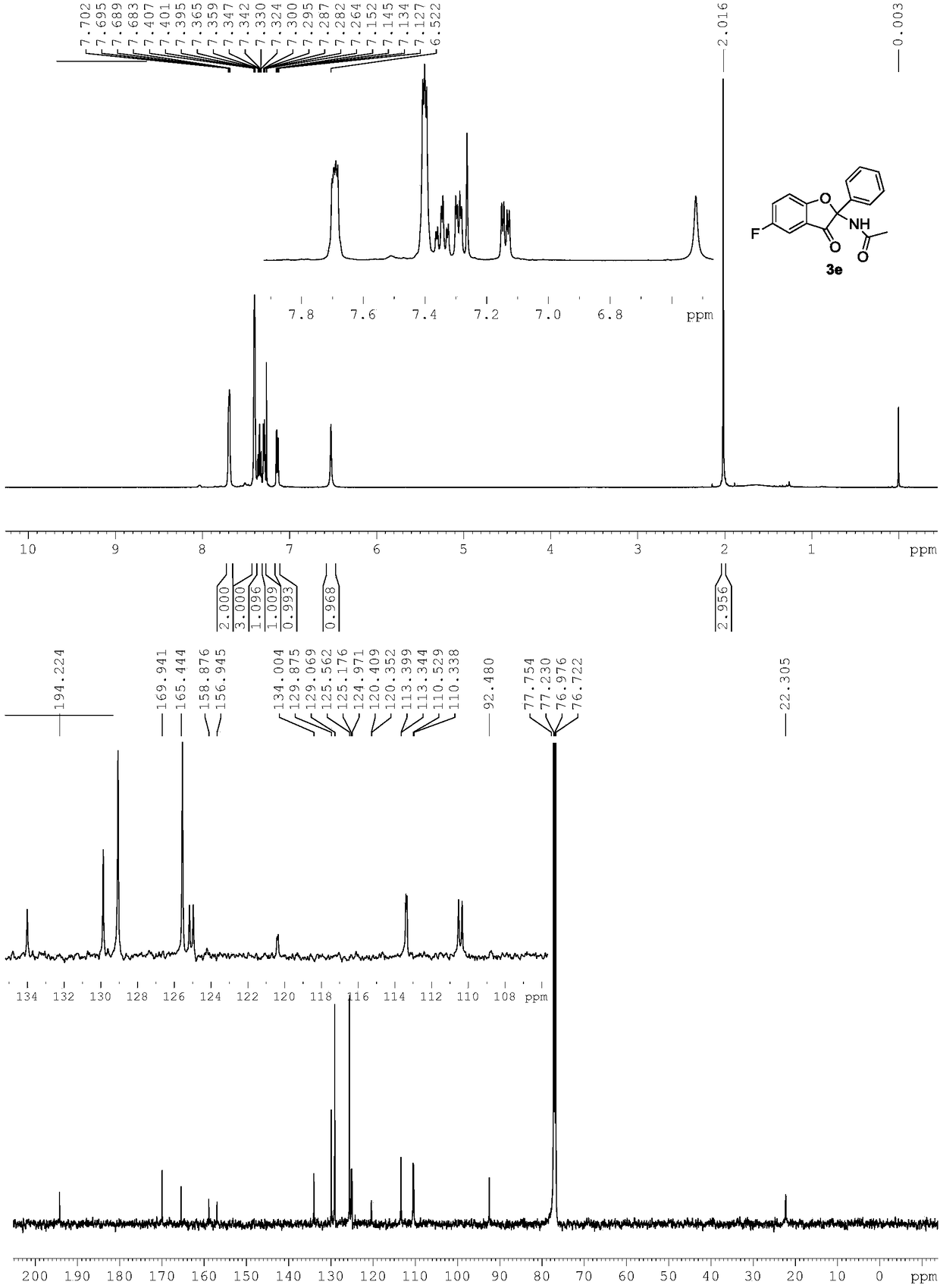
[0029] Use 1b to replace 1a in Example 1, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0030]
[0031] Spectral analysis data 3b:
[0032] 1 H NMR(500MHz, CDCl 3 ): δ7.70(brs,2H),7.44-7.38(m,5H), 7.08-7.07(m,2H), 6.96(brs,1H), 6.50(s,1H), 2.32(s,3H), 2.00(s,3H). 13 C NMR(125MHz, CDCl 3 ):δ194.9,169.8,167.8,138.9,134.6,132.0,129.6,128.9,125.6,124.6,119.6,111.9,91.7,22.4,20.6.HRMS(ESI-TOF,[M+Na] + ):calcd for C 17 H 15 NO 3 Na,304.0950,found 304.0951.
Embodiment 3
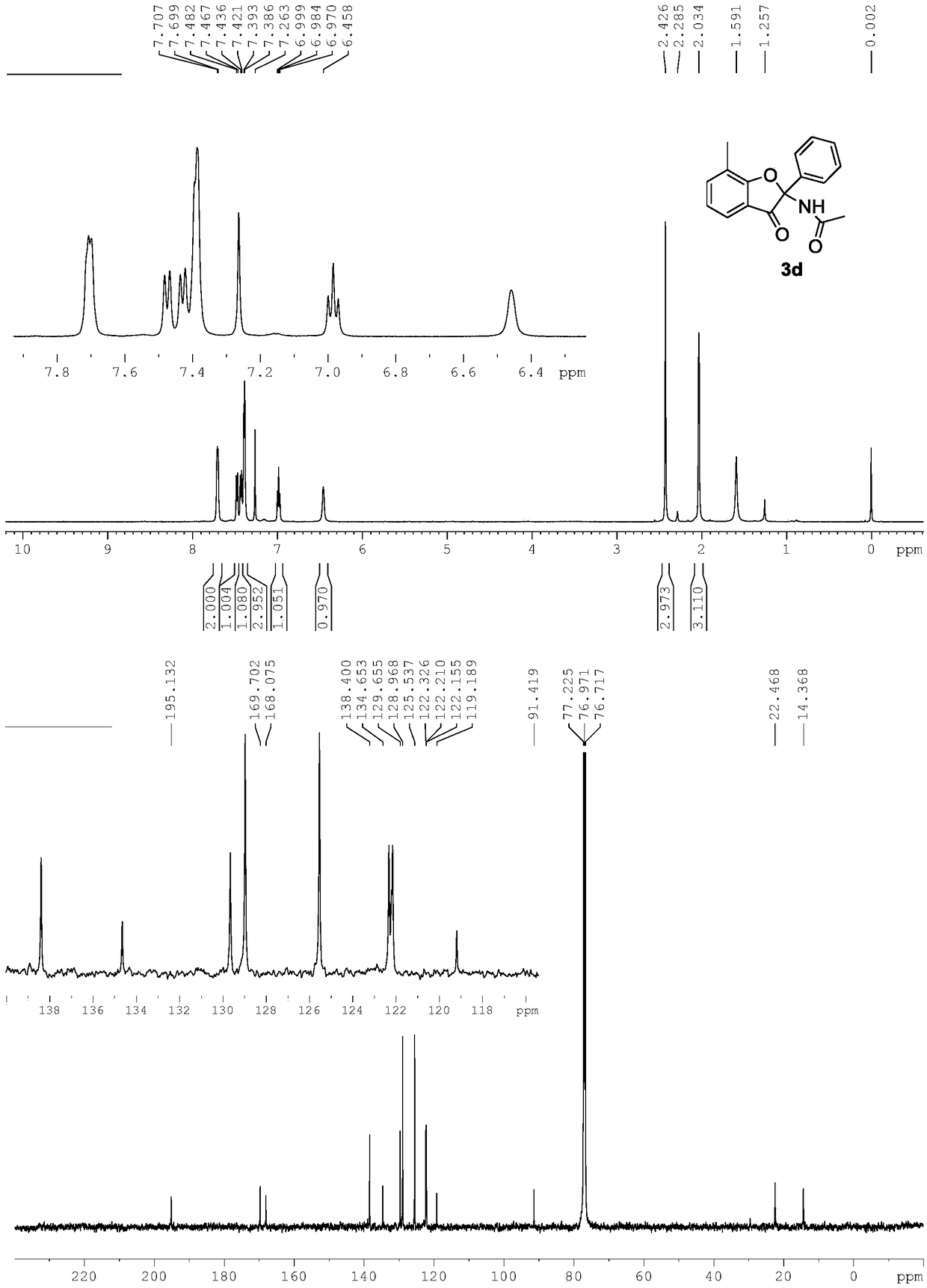
[0034] Replace 1a in Example 1 with 1c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0035]
[0036] Spectral analysis data 3c:
[0037] 1 H NMR(500MHz, CDCl 3 ): δ7.71-7.69(m,2H), 7.52(d,J=7.8Hz,1H), 7.38-7.37(m,3H), 6.98(s,1H), 6.90(d,J=7.8Hz, 1H), 6.51(s, 1H), 2.43(s, 3H), 1.99(s, 3H). 13 C NMR(125MHz, CDCl 3 ):δ194.1,169.9,169.8,149.8,134.8,129.6,128.9,125.5,124.8,123.8,117.4,112.4,91.8,22.5,22.4.HRMS(ESI-TOF,[M+Na] + ):calcd for C 17 H 15 NO 3 Na,304.0950,found 304.0952.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com