Method for oxidization pretreatment of zinc-containing secondary materials
A pretreatment and material technology, applied in the field of hydrometallurgy, can solve the problems of poor removal effect, complicated purification process, easy introduction of impurity ions, etc., and achieve the effect of improving removal effect, high purity, and shortening purification process steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
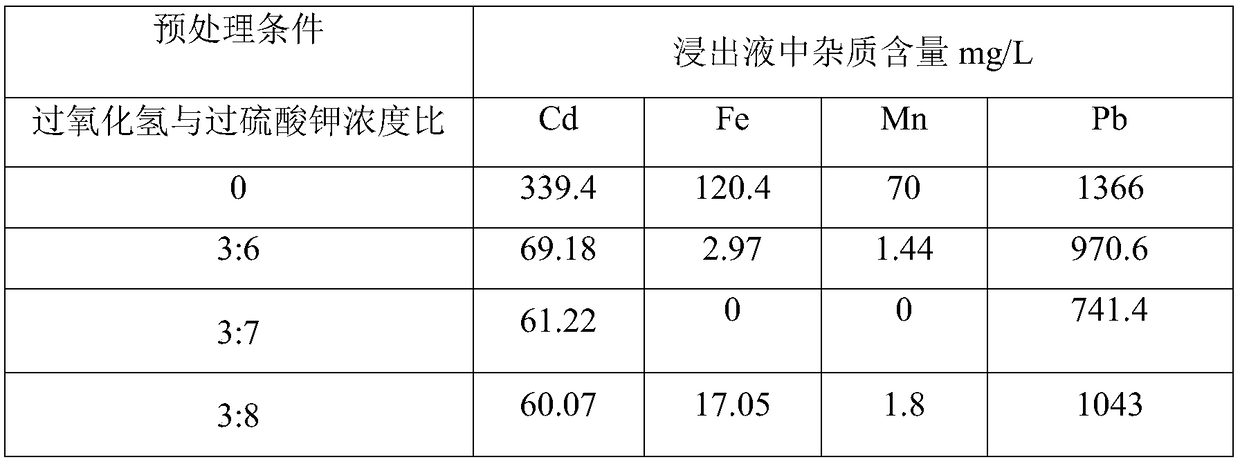
[0036] Take 25g of secondary zinc oxide for oxidation pretreatment, and select the molar concentration ratio of the double oxidant hydrogen peroxide and potassium persulfate to be 0, 3:6, 3:7, and 3:8, respectively. The ratio of the concentration of oxidant to the concentration of ferrous iron and manganese in secondary zinc oxide is 11. The reaction temperature was 60°C, the pretreatment time was 120min, and the natural pH of the reaction system was 6-7. After the pretreatment, the filter cake was filtered and dried at 90°C for 5 hours to obtain four groups of pretreated samples.
[0037] The pretreated samples of each group were soaked in ammonia at 30°C for 1 hour, and the zinc-ammonia leaching solution was obtained by filtration, and the content of iron, manganese, cadmium, and lead metal impurity elements in the leaching solution of each group was detected. The results are shown in Table 1:
[0038] Comparing the data of each group in Table 1, it can be seen that when th...
Embodiment 2
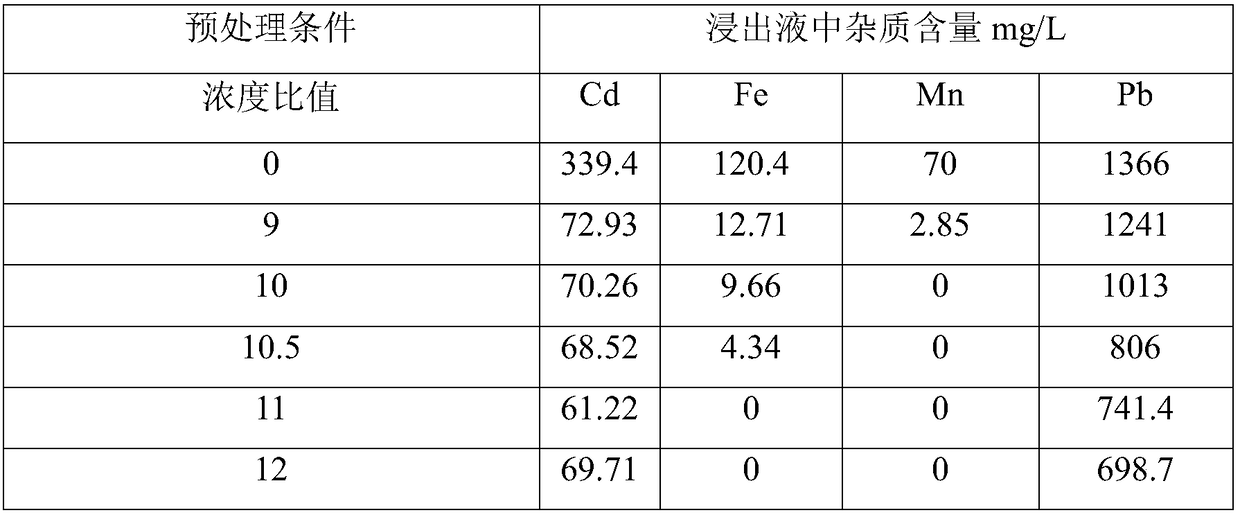
[0042] Get 25g secondary zinc oxide and carry out oxidative pretreatment, select the molar concentration ratio of hydrogen peroxide and potassium persulfate to be 3:7, get the concentration ratio of oxidant concentration and ferrous iron in secondary zinc oxide, the concentration ratio of manganese element to be 9,10 respectively, 10.5, 11, 12, with other steps unchanged, detect the content of iron, manganese, cadmium, and lead metal impurity elements in each group of leaching solutions, and the results are shown in Table 2:
[0043] Table 2 Contents of metal impurity elements in leachate
[0044]
[0045] It can be seen from Table 2 that when the ratio of the concentration of oxidant to the concentration of ferrous iron and manganese in secondary zinc oxide increases to 11, there is no iron and manganese in the leaching solution, and the concentrations of cadmium and lead are relatively low. smaller.
Embodiment 3
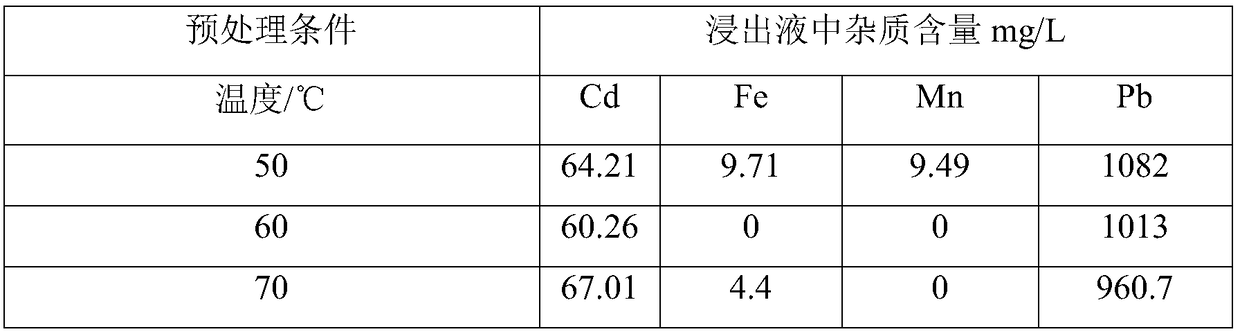
[0047] Get 25g secondary zinc oxide and carry out oxidative pretreatment, select the molar concentration ratio of hydrogen peroxide and potassium persulfate to be 3:7, the concentration ratio of oxidant concentration and secondary zinc oxide, ferrous iron, manganese element concentration ratio is 11, pretreatment temperature respectively Select 50°C, 60°C, and 70°C, and keep the other steps unchanged, and detect the content of iron, manganese, cadmium, and lead metal impurity elements in the leaching solutions of each group. The results are shown in Table 3:
[0048] It can be seen from Table 3 that when the pretreatment temperature increases to 60°C, the content of iron and manganese elements in the leaching solution is 0, and there is no iron and manganese elements in the leaching solution.
[0049] Table 3 Content of metal impurity elements in leachate
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com