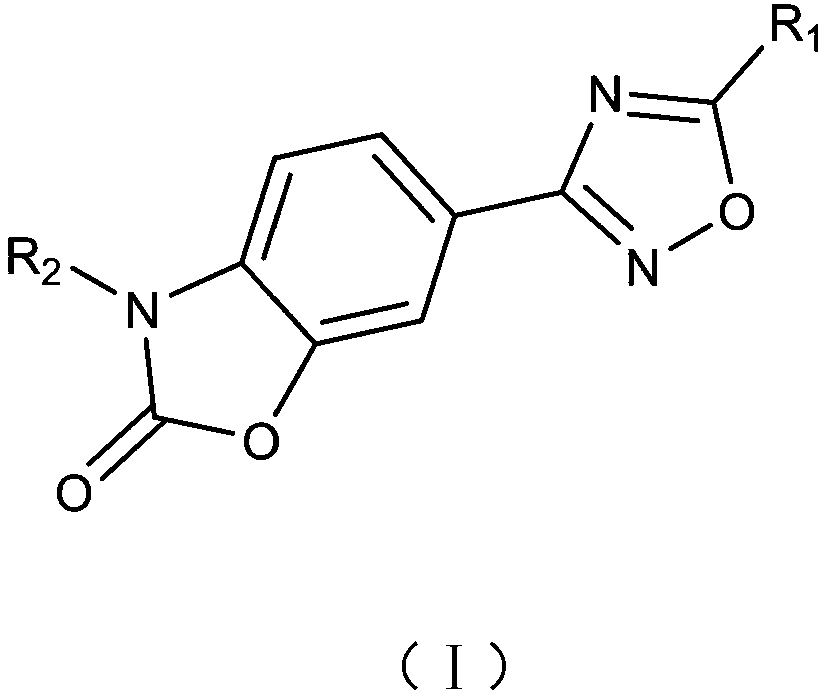
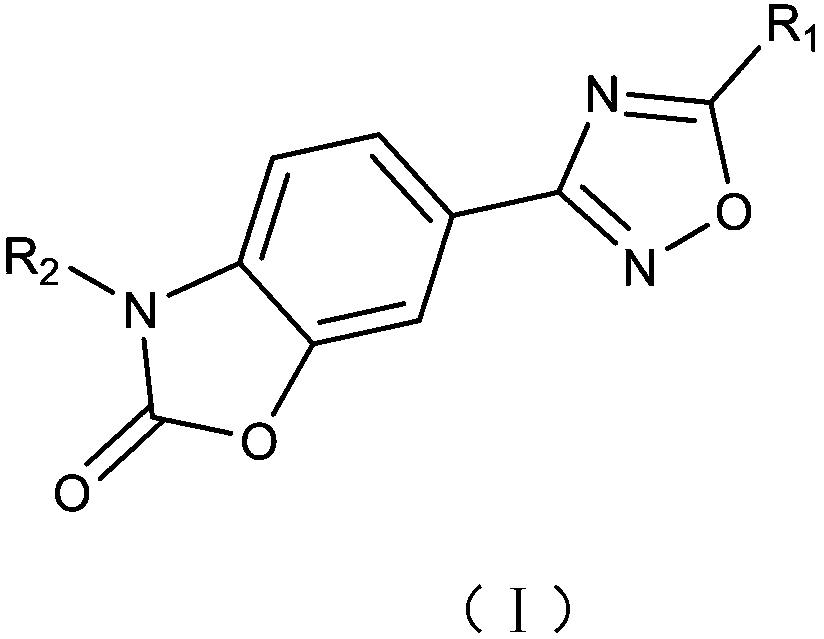
Small molecular compound for inhibiting PD-1/PD-L1 and application thereof
A PD-L1, PD-1 technology, applied in the field of medicine, can solve the problem of slow progress in the development of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
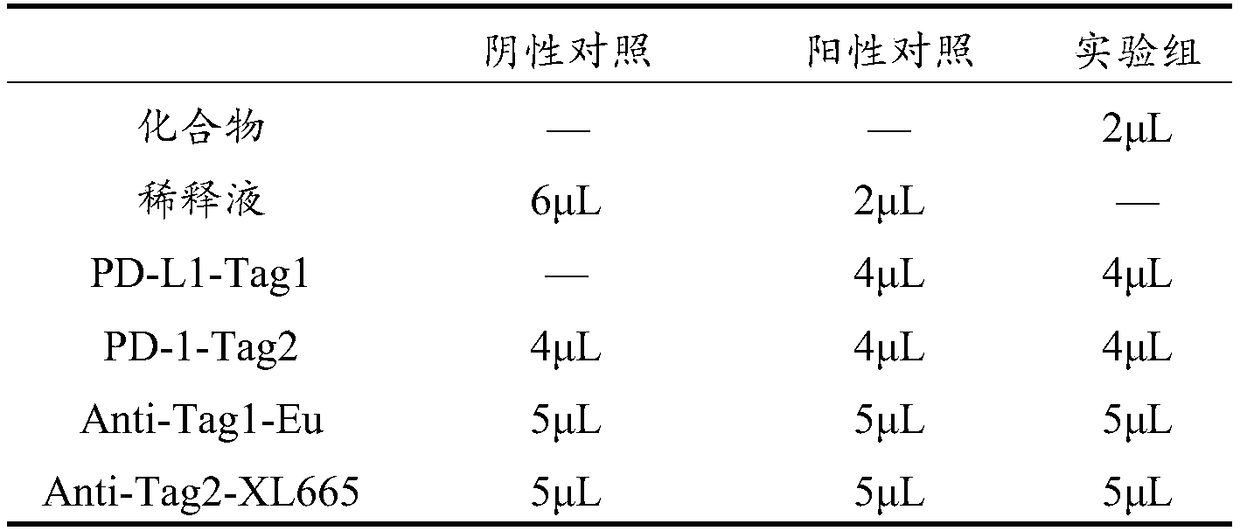
[0019] The present invention uses a commercial PD-1 / PD-L1 inhibitor screening kit, which is a high-throughput kit developed based on Homogeneous Time-Resolved Fluorescence (HTRF). Mainly rely on the excited rare earth metal chelate to release energy and transfer to the luminescent group to generate detection signal. In the experiment, Tag1-PD-1 with tag 1 will bind to Tag2-PD-L1 with tag 2, and the chelated Eu 3+ After the Tag1 antibody and the Tag2 antibody with the luminescent group XL665, the antibodies will bind to their respective target proteins. In the absence of blocking agents, PD-1 and PD-L1 bind to each other, and the two fluorescent groups are close to each other. , fluorescence resonance energy transfer occurs, and a part of the energy will be transferred from the fluorescence donor Eu 3+ The group transfers to the fluorescent acceptor XL665 group, and generates fluorescence at 665nm, while the energy that has not been transferred will emit fluorescence at 620nm,...
Embodiment 2
[0040] Example 2 Inhibitory effect of compounds M355-0148, M355-0149, and M355-0152 on mouse melanoma in vivo
[0041] Ⅰ. Cell Culture
[0042] B16-F10 cells were cultured in 1640 complete medium (containing 10% FBS), and when the cells were in the logarithmic growth phase and the growth confluence reached 80%-90%, they were passaged by trypsinization.
[0043] Ⅱ. Tumor transplantation experiment of mouse melanoma B16-F10
[0044] (1) Grouping of mice
[0045]A total of 24 mice with basically the same growth status were taken and divided into 4 groups, including the control group, M355-0148, M355-0149, and M355-0152 groups; -0152 is a drug, and the dosage is 20mg / kg, administered by intragastric administration.
[0046] (2) Mice were inoculated with melanoma B16-F10 cells intradermally
[0047] ① Anesthetize the mouse: Invert the EP tube containing the melanoma B16-F10 cells for 6 times and draw up the cell culture medium to 0.4ml;
[0048] ② B16F10 cells were intradermal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com