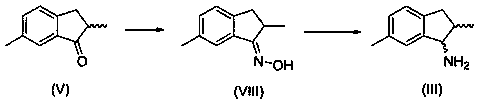
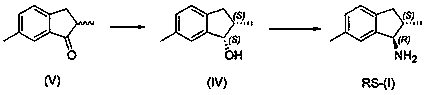
Preparation method of (1R, 2S)-2, 6-dimethyl-1-aminoindan
A technology of aminoindene and dimethyl, applied in the field of herbicide triazine indenazone synthesis, can solve the problems of low yield, inconvenient compound, low dr value and the like, and achieves the effects of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of 2,6-dimethyl-1-aminoindan and its salts
[0058] 1. 2,6-Dimethyl-1-aminoindane acetate (1-3)
[0059]
[0060] Dissolve 200g of 2,6-dimethyl-1-indanone (1-1) in 1000mL of ethanol solution, and quickly drop 2000mL of aqueous solution containing 307g of sodium acetate into the reaction system. Heat to reflux, TLC detects that the reaction is complete in 2-3 hours. The ethanol was concentrated to remove, the reaction liquid was extracted with ethyl acetate, and the combined organic matter was dried and concentrated to obtain a light yellow oily crude product.
[0061] In a 1000mL hydrogenation kettle, dissolve 240g of the above crude product in 600mL of methanol, add 24g of 10% Pd / C, 24mL of acetic acid, pressurize the hydrogen to 2.0MPa, heat to about 55°C, and react overnight. Remove the Pd / C catalyst by filtration, concentrate and recover methanol, and recrystallize the crude product with methanol to obtain 2,6-dimethyl-1-aminoindane aceta...
Embodiment 2
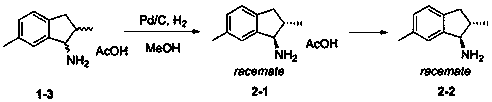
[0068] Example 2: Preparation of trans-2,6-dimethyl-1-aminoindan (2-1) by palladium-catalyzed hydrogenation
[0069]
[0070] Add 160 g of 6-dimethyl-1-aminoindane acetate (1-3) (the ratio of trans and cis isomers is 60:40) into 1.6 L of anhydrous methanol, add 5% Pd / C (Hangzhou Lianzhi) 16g, the reaction solution was pressurized to 35atm under the atmosphere of hydrogen, and reacted at 80-90°C for 48 hours, after the reaction was completed, it was cooled to room temperature, the palladium carbon catalyst was removed by filtration, and the filtrate was spin-dried to obtain 160g Trans-2,6-dimethyl-1-aminoindane (2-1) oil, detected by HPLC, 2,6-dimethyl-1-aminoindane acetate (2-1 ) in the ratio of trans to cis isomers is 85:15.
[0071] Table 1: The reaction results of trans-2,6-dimethyl-1-aminoindan prepared by palladium-catalyzed hydrogenation under different substrate and catalyst conditions
[0072]
Embodiment 3
[0073] Example 3: Preparation of trans-2,6-dimethyl-1-aminoindan (2-2) using the recovered isomer
[0074]
[0075] Free the waste isomers recovered in each step to obtain 50 g of 6-dimethyl-1-aminoindan (1-5) (RS-(I), SR-(I), RR-(I ) to the four isomers of SS-(I) in the ratio of 20:70:5:5) was added to 500mL of anhydrous methanol, and 5% Pd / C (Hangzhou Lianzhi) was added, and the reaction solution was pressurized to 30atm, and reacted at 110°C for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, the palladium carbon catalyst was removed by filtration, and the filtrate was spin-dried to obtain 50g trans-2,6-dimethyl-1-aminoindan ( 2-2) Oil, detected by chiral HPLC, RS-(I), SR-(I), RR-(I) in the product 2,6-dimethyl-1-aminoindan (2-2) The ratio with the four isomers of SS-(I) is 42:43:7:8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com