Eutectic of nifedipine and isonicotinamide
A technology of nifedipine and isonicotinamide, which is applied in the field of co-crystal of nifedipine and isonicotinamide and its preparation, can solve problems such as skin damage and generation of impurities, achieve easy crystallization process, good reproducibility, and preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Co-crystal of nifedipine and isonicotinamide
[0067] At room temperature, form a saturated solution of isonicotinamide (12.2g) in 200mL of methyl isobutyl ketone solution, filter and take out the supernatant, add nifedipine (46.1g) powder to it, and suspend until it forms Supersaturated state, centrifugation and filtration, to obtain the co-crystal of nifedipine and isonicotinamide (33.6g). The prepared co-crystal was characterized by X-ray single crystal diffraction. The X-ray single crystal diffraction structure diagram of the co-crystal of nifedipine and isonicotinamide is shown in figure 1 , The results of X-ray single crystal diffraction showed that the molar ratio of nifedipine and isonicotinamide was 1:1. The crystal form of the co-crystal of nifedipine and isonicotinamide is monoclinic, and the space group is P2 1 / n, the cell parameters are: α=90°; β=90.080(5)°; γ=90°, the unit cell volume is
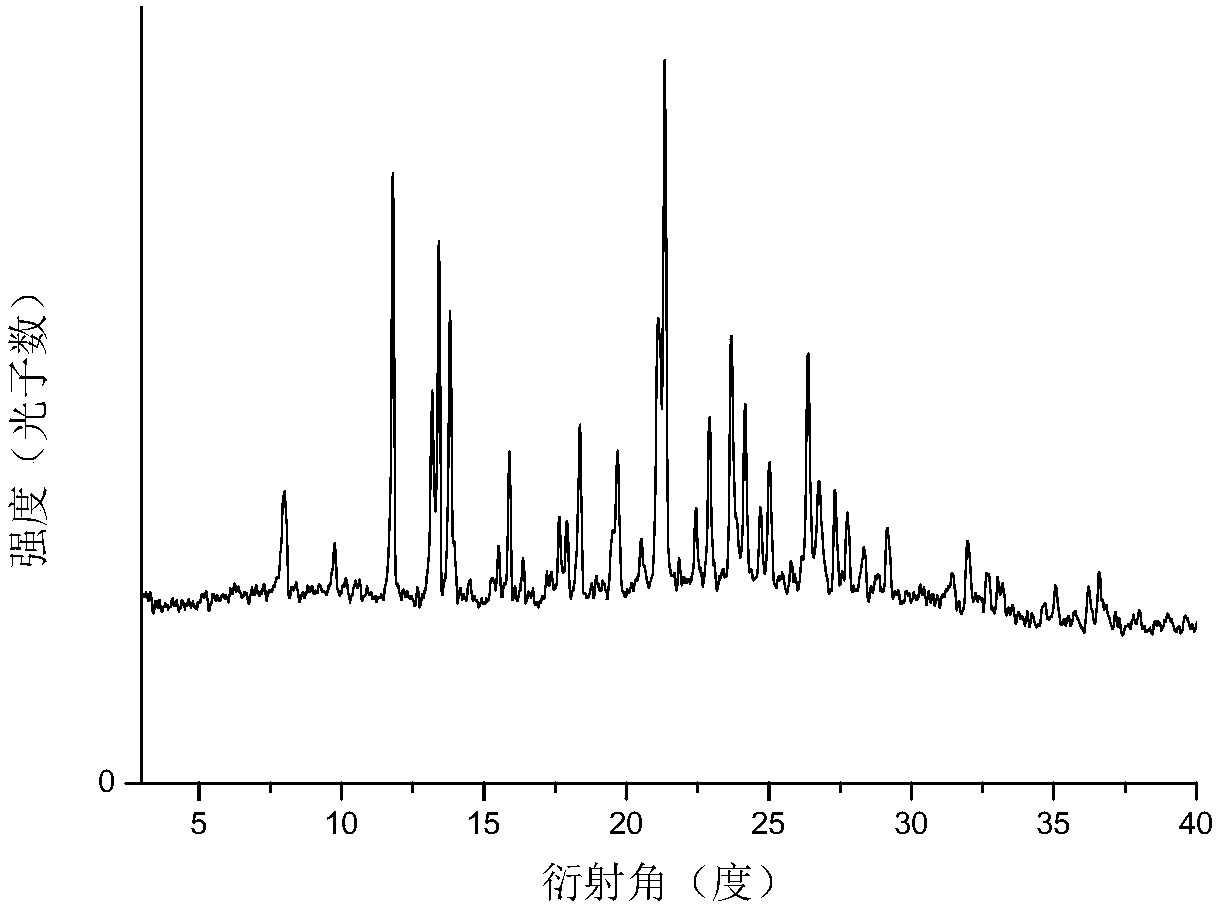
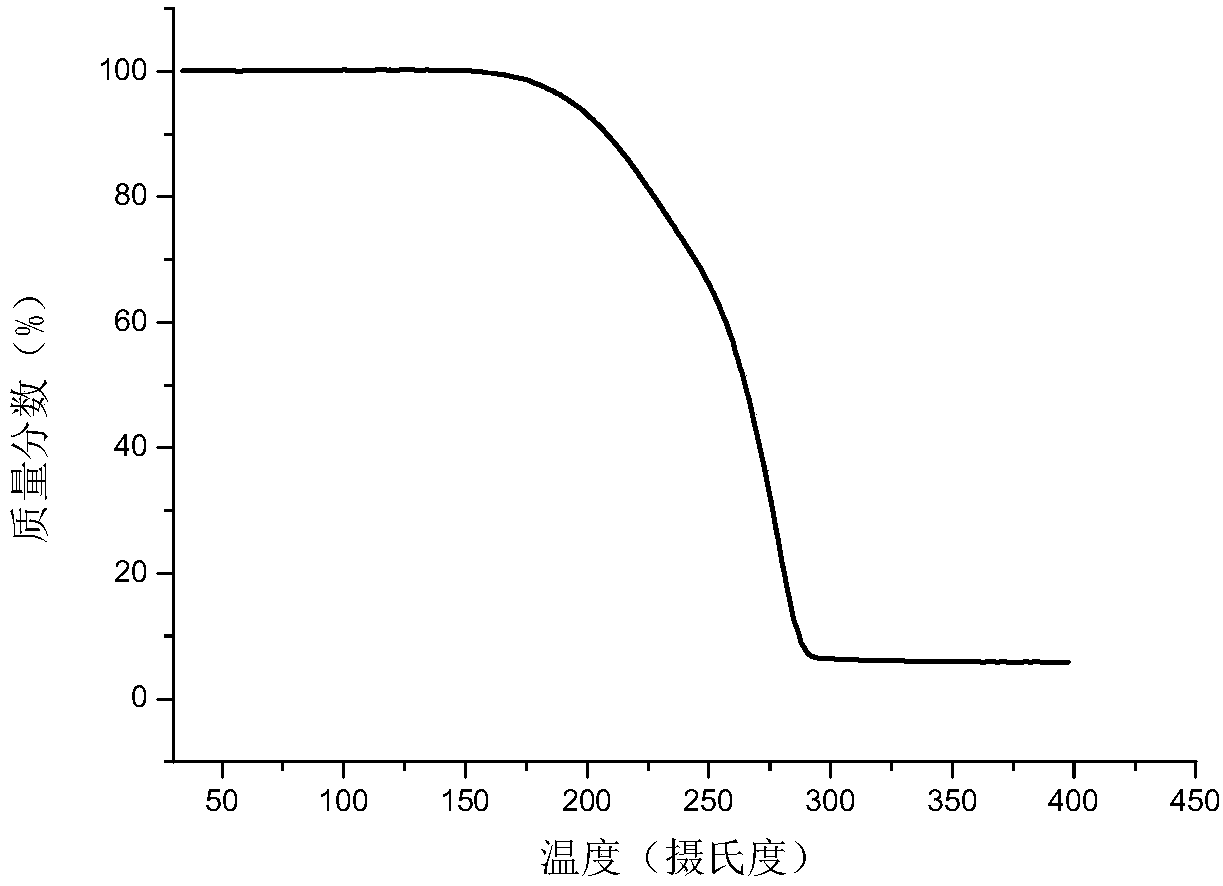
[0068] Also adopt X-ray powder diffraction (XRPD), therm...
Embodiment 2
[0070] Co-crystal of nifedipine and isonicotinamide
[0071] At room temperature, form a saturated solution of nifedipine (34.6g) in 200mL of methyl isobutyl ketone solution, filter the supernatant, add isonicotinamide (12.2g) powder to it, and suspend until it forms Supersaturated state, centrifugation and filtration to obtain a co-crystal of nifedipine and isonicotinamide (35.1 g).
Embodiment 3
[0073] Co-crystal of nifedipine and isonicotinamide
[0074] At room temperature, form a saturated solution of isonicotinamide (30.5g) in 250mL of methanol solution, filter and take out the supernatant, add nifedipine (86.5g) powder to it, suspend until supersaturated state is formed, centrifuge and filtration to obtain a co-crystal of nifedipine and isonicotinamide (90.6 g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com