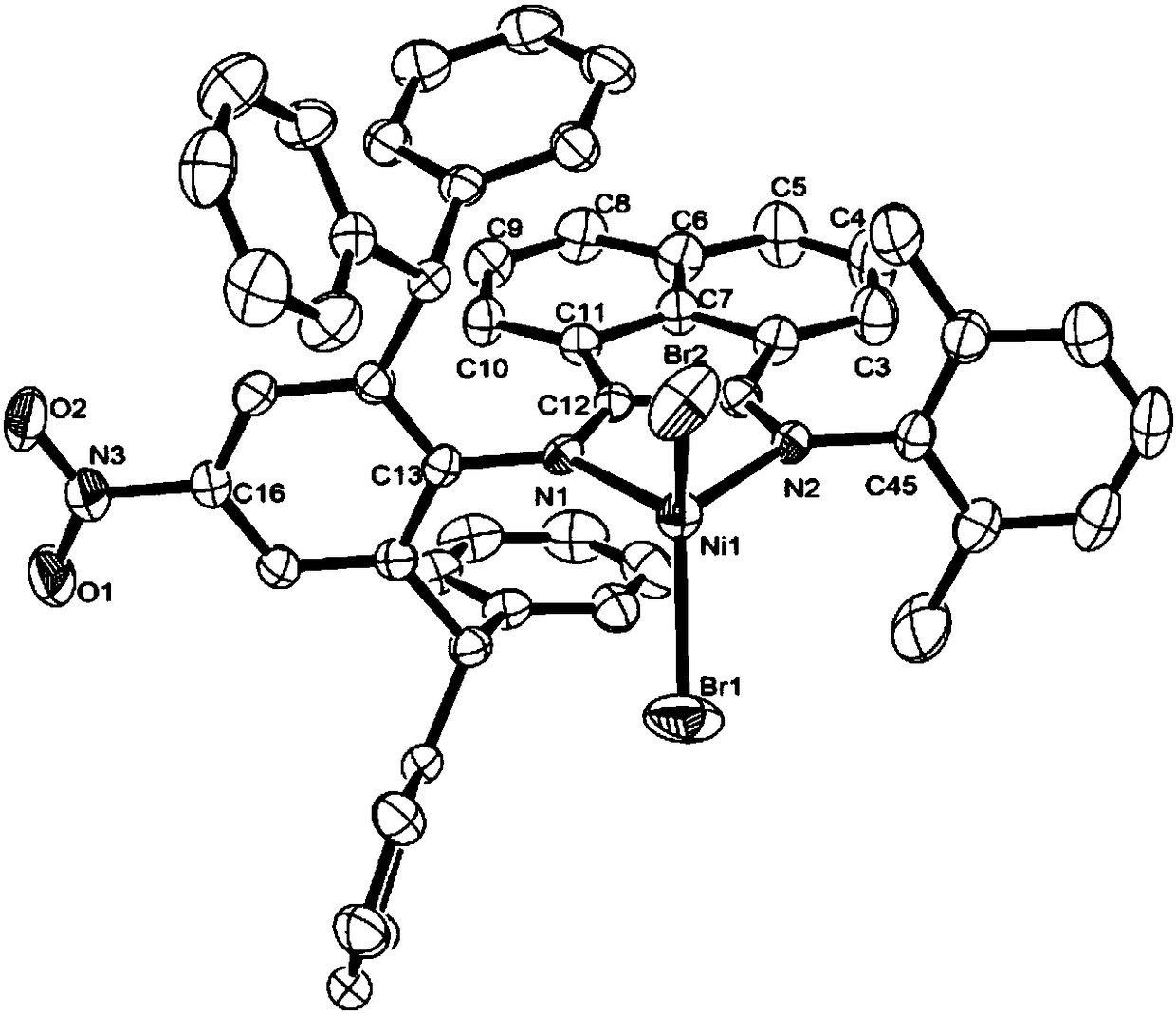
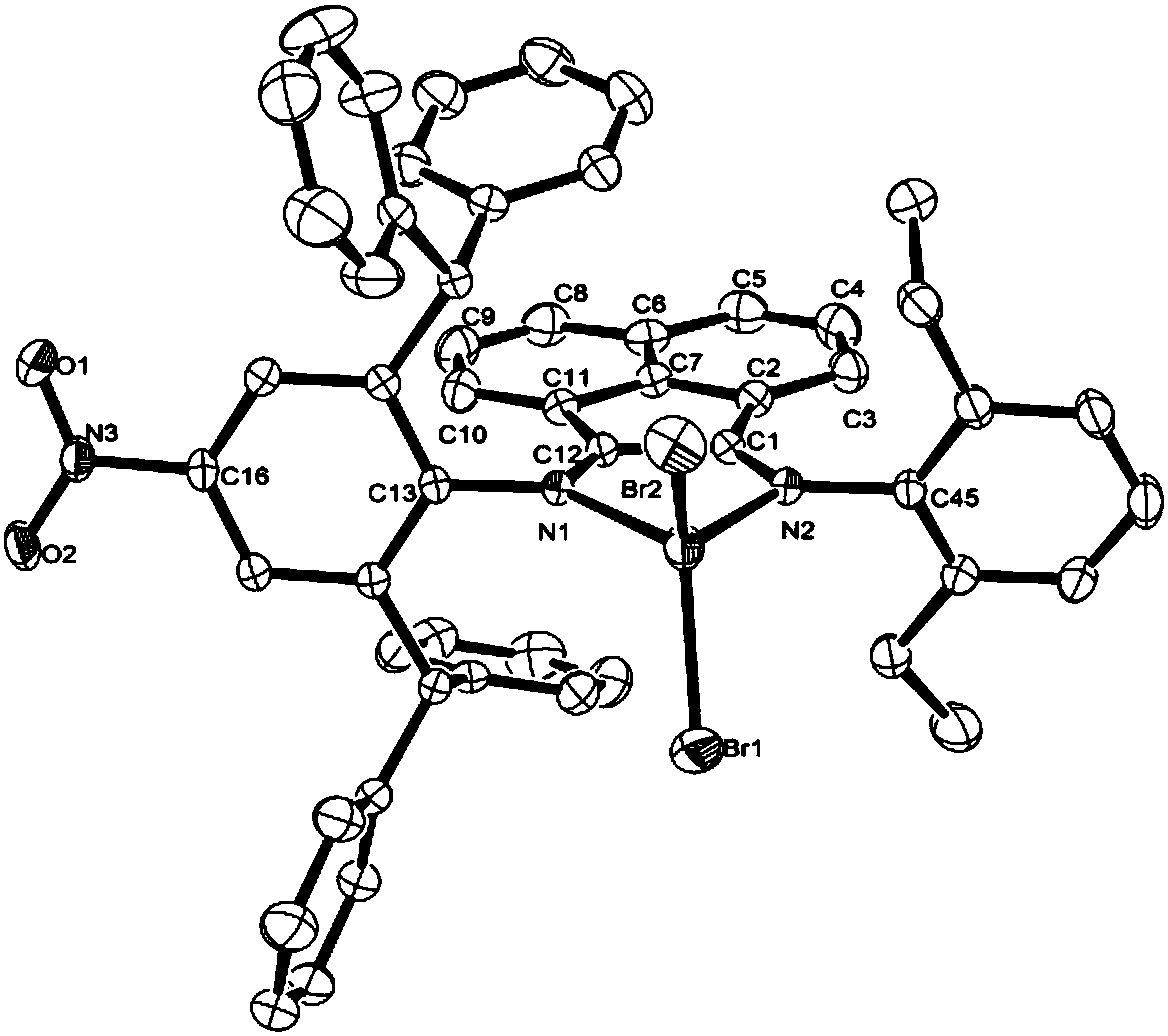
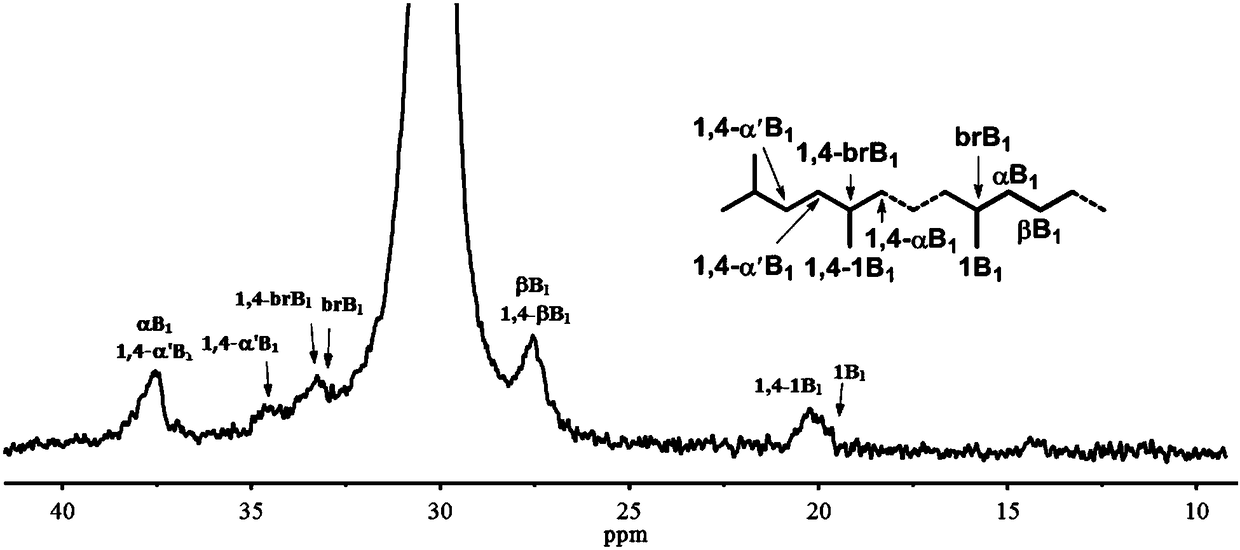
Nitryl asymmetric alpha-diimine nickel complex for preparation of ultrahigh-molecular-weight polyethylene and preparation method and application of nitryl asymmetric alpha-diimine nickel complex
A technology of nickel complexes and nickel diimides, applied to nickel organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] 2-(2,6-bis(benzhydryl)-4-nitroanilino)acenaphthenone represented by formula (V) was prepared.
[0128] Add catalyst amount (1.25g) of p- Toluenesulfonic acid, reflux reaction for 5h. The solvent was removed, and the residue was subjected to silica gel column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50, and the eluted fraction was detected through a thin-layer silica gel plate, and the developing solvent was the volume of petroleum ether and ethyl acetate. A mixed solvent with a ratio of 10:1, the third fraction was collected, and an orange-yellow solid was obtained after removal of the solvent. Yield: 70%.
[0129] The structural confirmation data are as follows:
[0130] 1 H NMR (400MHz, DMSO, TMS): δ8.32(d, J=8.8Hz, 1H), 8.18(d, J=6.8Hz, 1H), 7.86-7.82(m, 2H), 7.69-7.52(m ,3H),7.32(t,J=7.6Hz,1H),7.26-6.92(m,20H).
[0131] 13 C NMR (100MHz, CDCl 3 , TMS): δ189.1, 161.2, 154.1, 148.3, 144.3, 142.8, 141.0, 14...
Embodiment 2
[0133] 1-(2,6-dimethylaniline)-2-(2,6-bis(benzhydryl)-4-nitroaniline) acenaphthene [L1] shown in preparation formula (II), wherein R 1 is methyl, R 2 for hydrogen.
[0134] 2-(2,6-bis(benzhydryl)-4-nitroaniline)acenaphthylenone (0.98g, 1.55mmol) and 2,6-dimethylaniline (0.2g, 1.65mmol) in toluene (100mL ) into the solution, add a catalytic amount of p-toluenesulfonic acid, and heat to reflux for 10h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50. The eluted fractions were examined through a thin-layer silica gel plate, the second fraction was collected, and the solvent was removed to give an orange-yellow solid. Yield: 23%. Melting point: 162-164°C.
[0135] The structural confirmation data are as follows:
[0136] FT-IR (KBr, cm -1):3057(w), 1661(ν(C=N),w), 1589(w), 1494(m), 1467(s), 1408(w), 1294(vs), 1182(m), 1071 (m), 1027(m), ...
Embodiment 3
[0141] 1-(2,6-diethylaniline)-2-(2,6-bis(benzhydryl)-4-nitroaniline) acenaphthene [L2] shown in preparation formula (II), wherein R 1 is ethyl, R 2 for hydrogen.
[0142] 2-(2,6-bis(benzhydryl)-4-nitroaniline)acenaphthylenone (0.98g, 1.55mmol) and 2,6-diethylaniline (0.21g, 1.65mmol) in toluene (100mL ) into the solution, add a catalytic amount of p-toluenesulfonic acid, and heat to reflux for 10h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50. The eluted fractions were examined through a thin-layer silica gel plate, the second fraction was collected, and the solvent was removed to give an orange-yellow solid. Yield: 41%. Melting point: 170-172°C.
[0143] The structural confirmation data are as follows:
[0144] FT-IR (KBr, cm -1 ):3058(w), 2960(w), 1656(ν(C=N),w), 1587(w), 1498(m), 1470(m), 1447(s), 1340(m), 1290 (vs), 1184(m), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com