Preparation method of amphipathic star-shaped block polymer with ultra-high pH stimulus-response
A technology of star-shaped polymer and block polymer is applied in the directions of non-active components of polymer compounds, medical preparations and pharmaceutical formulations of non-active components, etc., which can solve the problems of poor stability of nano-micelles and low drug loading capacity. , to achieve the effect of improving micelle stability and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments.
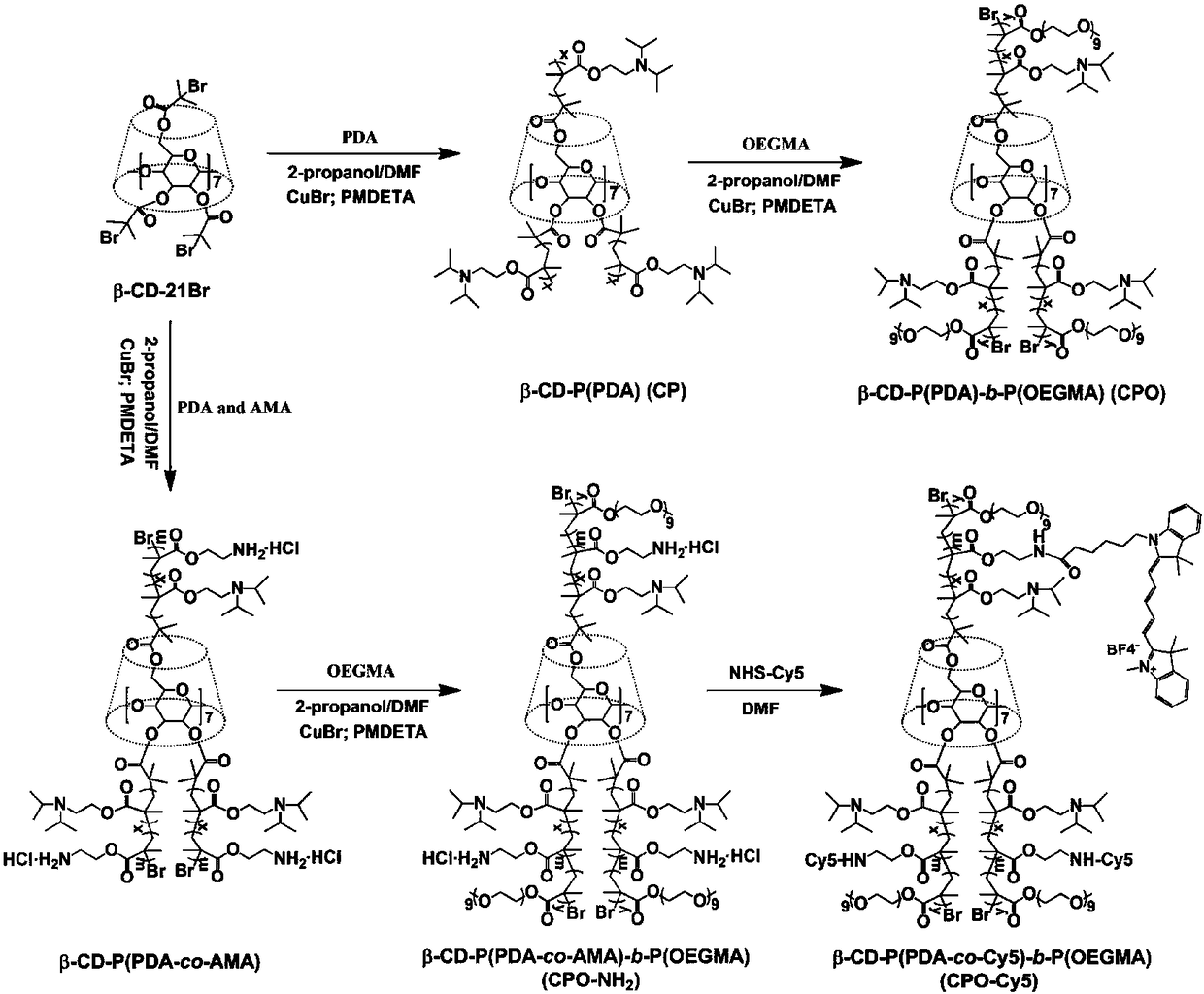
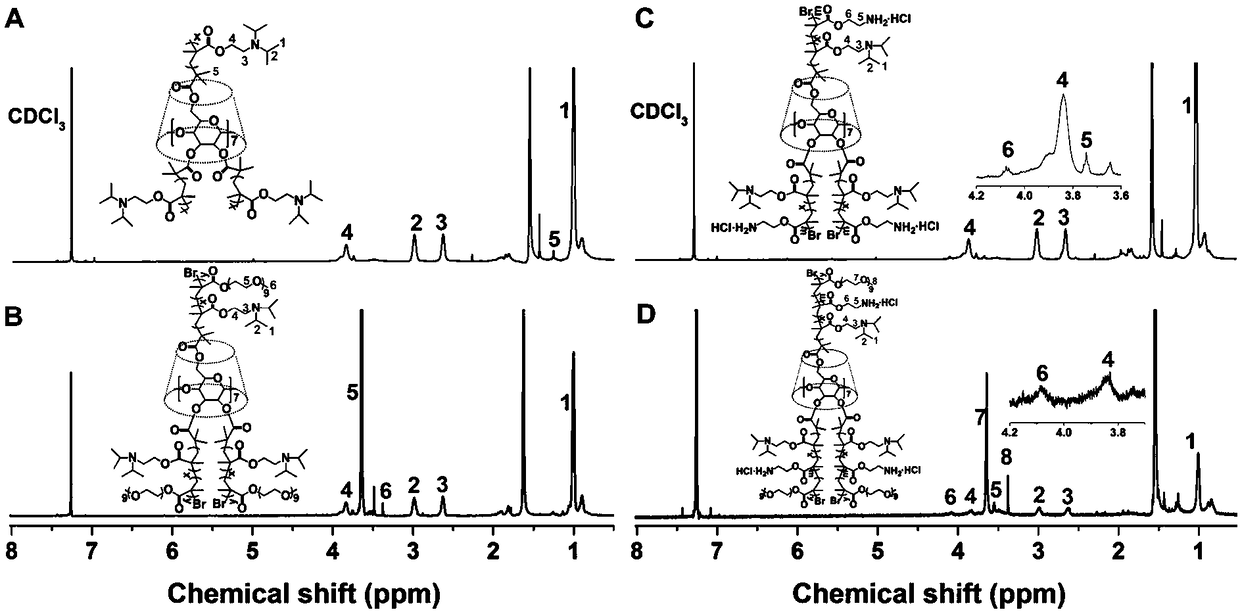
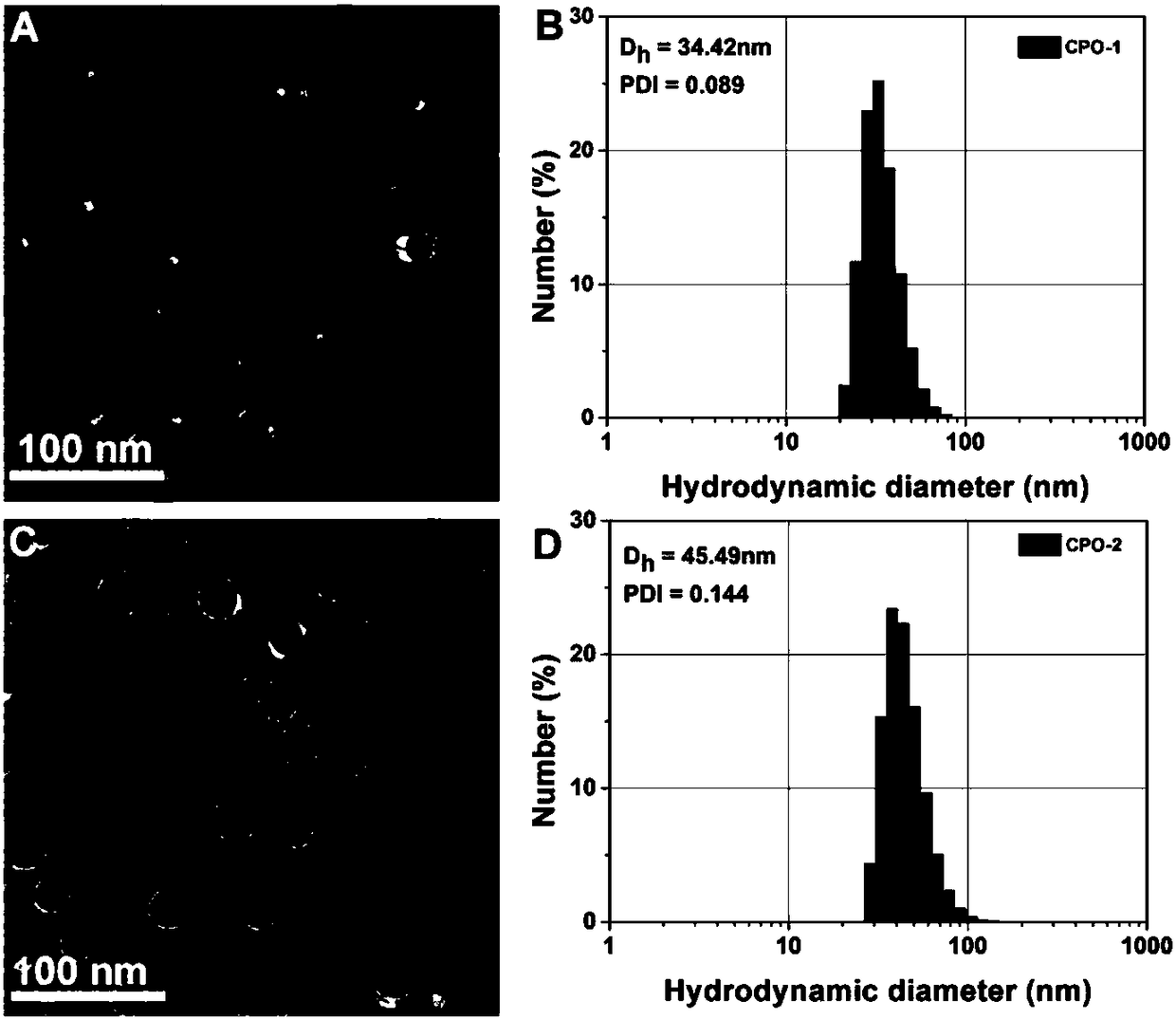
[0026] Such as Figure 1 to Figure 5 Shown, a method for preparing an ultrahigh pH stimuli-responsive amphiphilic star-shaped block polymer comprises the following two parts:
[0027] The first part: Preparation of pH-sensitive β-CD-PPDA-POEGMA amphiphilic star polymer; this part is mainly used to synthesize amphiphilic materials to embed hydrophobic drugs, so as to realize the function and benefits of being a drug carrier.
[0028] The specific steps are as follows:
[0029] In the first step, cyclodextrin-based star atom radical polymerization (ATRP) initiator (CD-Br) was prepared: cyclodextrin (CD) and 2-bromoisobutyryl bromide were dissolved in 1-methyl React in pyrrolidone (NMP) for 48 hours, and purify to obtain the cyclodextrin (CD)-based star atomic radical polymerization (ATRP) initiator (CD-Br);
[0030]The second step is to prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com