Ullmann C-N cross-coupling reaction catalyst and synthesis method based on catalyst
A technology of cross-coupling reaction and synthesis method, applied in the field of catalysis, can solve the problems of insufficient catalytic activity, harsh reaction conditions, long reaction time, etc., and achieve the effects of improving the conversion rate of raw materials, high selectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The synthetic method based on above-mentioned Ullmann C-N cross-coupling catalyst comprises the following steps,
[0030] Step 1, under the protection of argon, add aromatic amine, halogenated hydrocarbon, copper source, potassium carbonate, mixed organic ligands and DMF into the container, and heat to 150°C-180°C under the protection of argon for reflux reaction for 1-6h ;
[0031]Step 2, after the reaction is completed, filter while it is hot, and rinse the obtained solid with DMF; then, add water dropwise to the above-mentioned DMF filtrate under high-speed stirring, and a large amount of off-white solid precipitates; filter the solid, wash with water, and dry;
[0032] Step 3, the above off-white solid was separated by silica gel column chromatography, the eluent was petroleum ether, and the eluate was collected, concentrated and dried to obtain a light white solid.
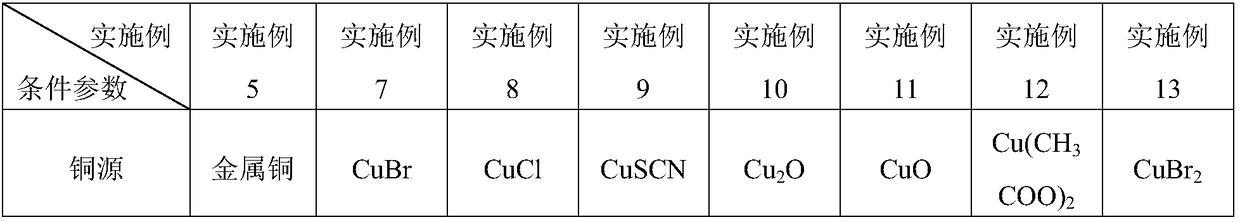
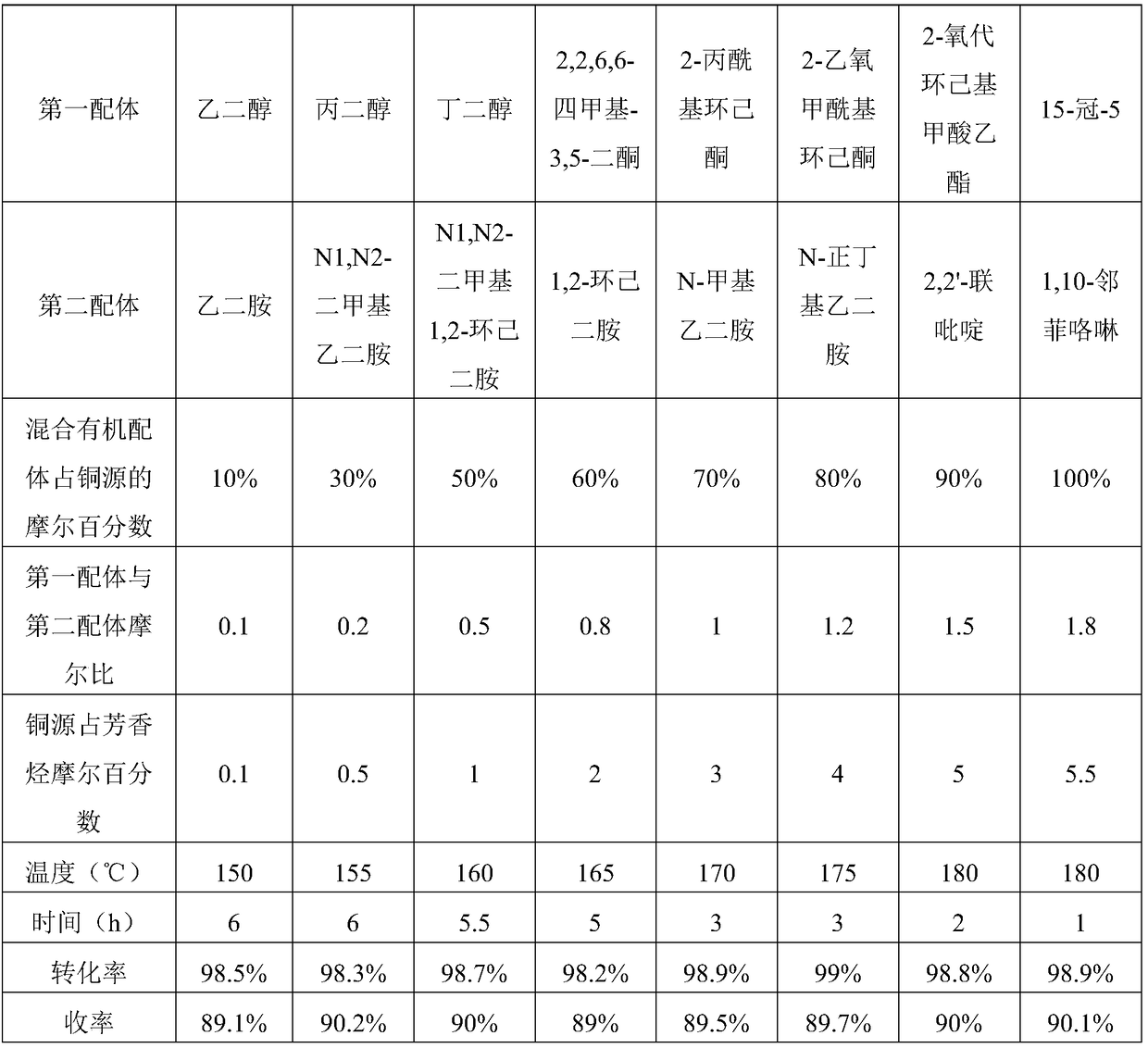
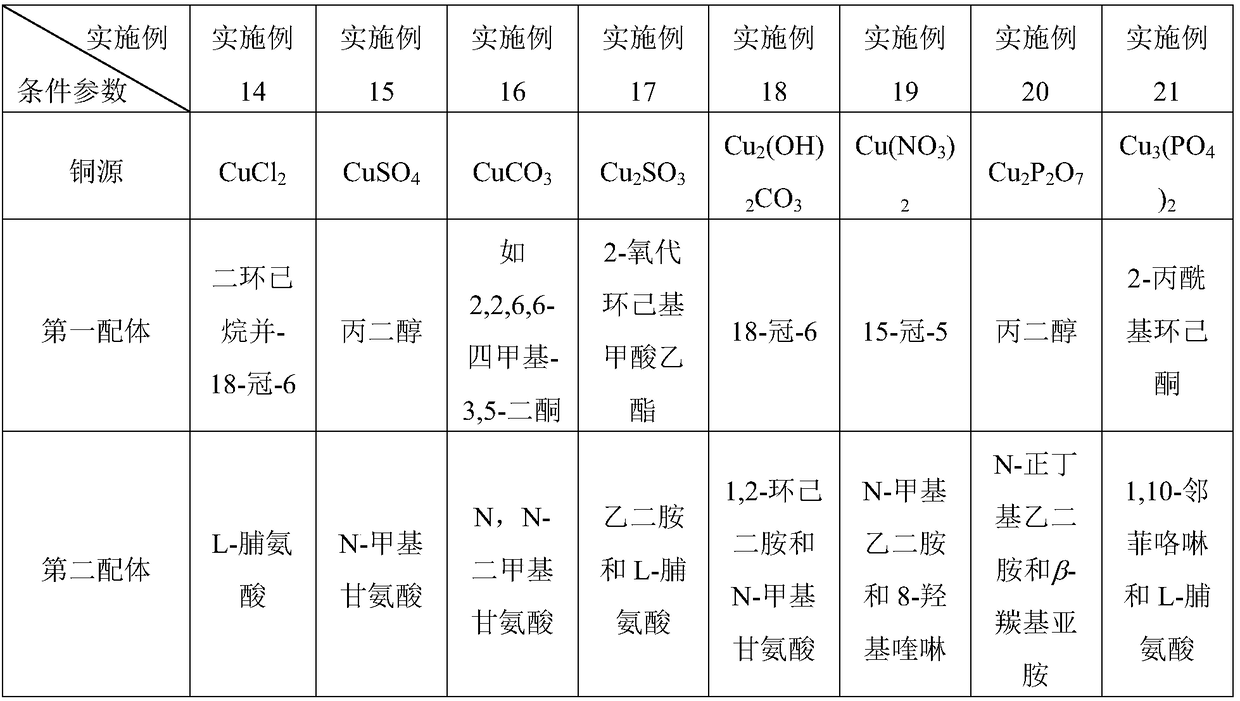
[0033] Wherein, in step 1, the amount of copper source is 0.1-10% of the amount of halogenated hyd...
Embodiment 1
[0036] Under argon protection, 1.67g (10mmol) carbazole, 1.88g (12mmol) bromobenzene, 0.382g (2mol) cuprous iodide, 2.76g (20mmol) potassium carbonate, 0.113g ( 0.5mmol) of 18-crown-6, 0.078g (0.5mmol) of 2,2'-bipyridine and 25mL of DMF, heated to 156°C under argon protection for 2h under reflux. After the reaction was completed, a sample was taken for analysis. The conversion rate of the reaction was 99%. It was filtered while it was hot, and the solid was rinsed with 10 mL of DMF. Then, 30 mL of water was added dropwise to the above DMF filtrate under high-speed stirring, and a large amount of off-white solids were precipitated. The solid was filtered, washed with water and dried. The above off-white solid was separated by silica gel column chromatography, the eluent was petroleum ether, and the eluate was collected, concentrated and dried to obtain 2.19 g of off-white solid, with a yield of 90%. m.p.91-95°C; IR, ν, cm-1: 483, 563, 626, 749, 846, 1022, 1171, 1230, 1337, 13...
Embodiment 2
[0038] Under argon protection, 1.67g (10mmol) carbazole, 1.88g (12mmol) bromobenzene, 0.382g (2mol) cuprous iodide, 2.76g (20mmol) potassium carbonate, 0.226g ( 1mmol) of 18-crown-6 and 25mL of DMF were heated to 156°C under the protection of argon for 2h under reflux. After the reaction was completed, a sample was taken for analysis, and the conversion rate was measured to be 87%, filtered while hot, and the solid was rinsed with 10 mL of DMF. Then, 30 mL of water was added dropwise to the above DMF filtrate under high-speed stirring, and a large amount of off-white solids were precipitated. The solid was filtered, washed with water and dried. The above off-white solid was separated by silica gel column chromatography, the eluent was petroleum ether, and the eluate was collected, concentrated and dried to obtain 1.95 g of off-white solid, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com