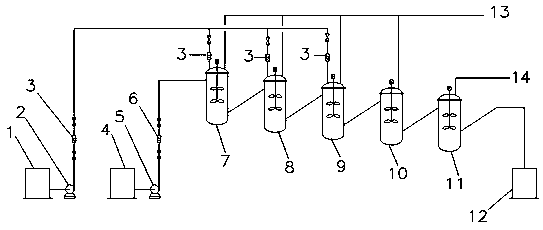
Dinaphthol production continuous neutralization reaction method
A dinaphthol and reaction technology, which is applied in the field of continuous neutralization reaction for the production of dinaphthol, can solve the problems of poor operating environment, harsh operating environment, leakage of sulfur dioxide into workshop air, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] During the above-mentioned whole neutralization reaction process, the molar ratio of sodium sulfite solution to naphthalenesulfonic acid is: 97:100.
[0042] The liquid mixture obtained after the five-stage neutralization reaction has a solution pH value of 1.5.
[0043] The internal neutralization reaction temperature in the neutralization reactor of each stage is 95°C.
[0044] The primary sodium sulfite solution: the secondary sodium sulfite solution: the mass ratio of the three sodium sulfite solutions is 5:4:2.
Embodiment 2
[0046] During the above-mentioned whole neutralization reaction process, the molar ratio of sodium sulfite solution to naphthalenesulfonic acid is: 95:100.
[0047] The liquid mixture obtained after the five-stage neutralization reaction has a solution pH value of 1.
[0048] The internal neutralization reaction temperature in the neutralization reactor of each stage is 98°C.
[0049] The mass ratio of the primary sodium sulfite solution: the secondary sodium sulfite solution: the tertiary sodium sulfite solution is 1:1:1.
Embodiment 3
[0051] During the above-mentioned whole neutralization reaction process, the molar ratio of sodium sulfite solution to naphthalenesulfonic acid is: 98:100.
[0052] The liquid mixture obtained after the five-stage neutralization reaction has a solution pH value of 2.
[0053] The internal neutralization reaction temperature in the neutralization reactor of each stage is 100°C.
[0054] The mass ratio of described primary sodium sulfite solution: secondary sodium sulfite solution: tertiary sodium sulfite solution is 3:2:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com