Antibacterial ultraviolet absorbent as well as preparation method and application thereof in polymer
An absorber and ultraviolet technology, which is applied in the preparation of carboxylic acid amide, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of destruction and aging of synthetic polymer materials, and achieve the effect of easy operation and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of 3,5-di-N-methylbenzamide-2,4-dihydroxybenzophenone in ethanol-concentrated sulfuric acid system:
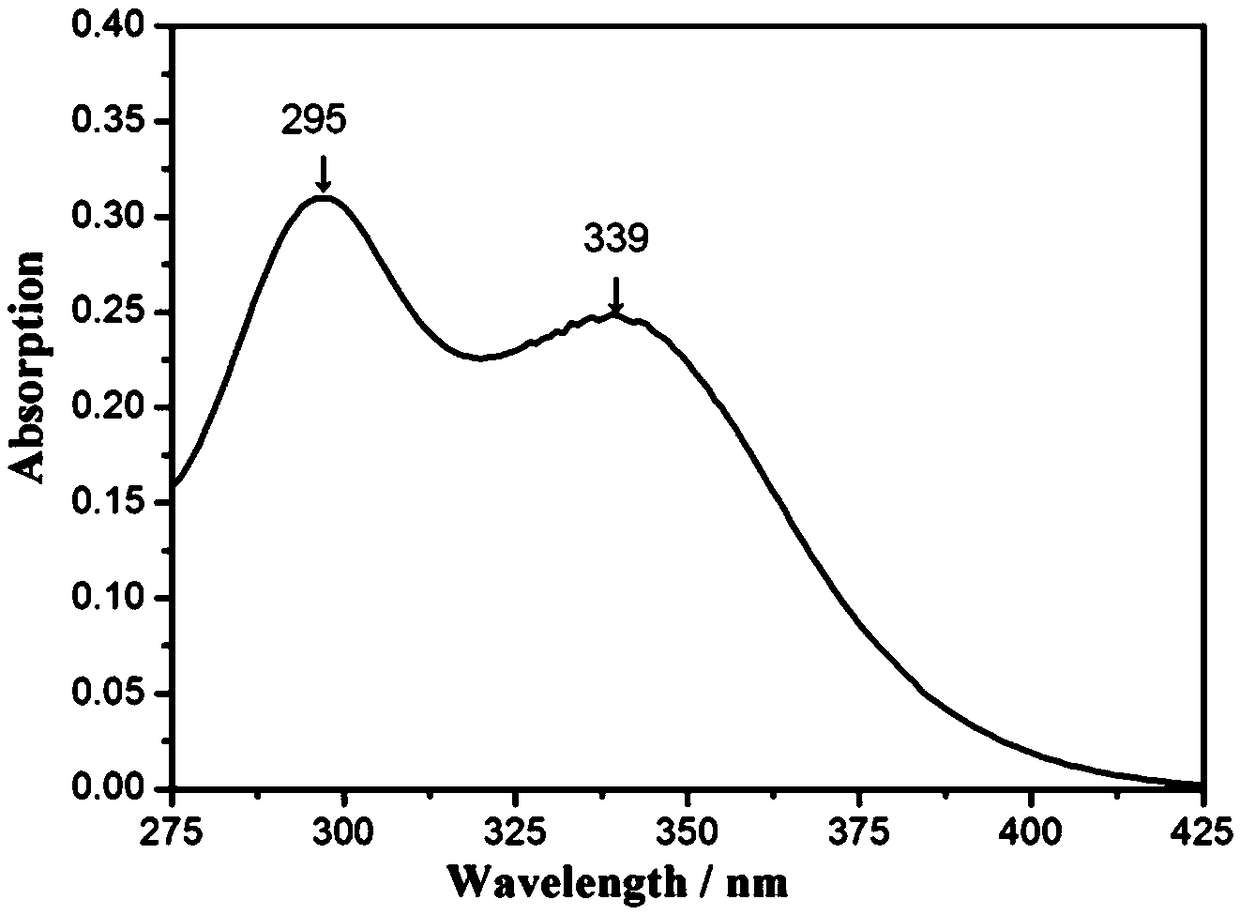
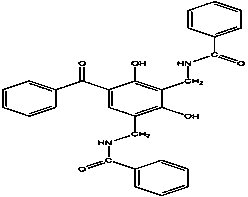
[0031] Add 21.4g (0.1mol) of 2,4-dihydroxybenzophenone, 20.2g (0.1mol) of N-methylolbenzamide and 100mL of anhydrous Ethanol, heated and stirred in an oil bath at 35°C, and then added dropwise 5mL of concentrated sulfuric acid as a catalyst. After 3 days, the reaction was completed. After cooling to room temperature, the solid-liquid separation was carried out, and the obtained solid was washed repeatedly with distilled water until neutral, and weighed with absolute ethanol. Crystallized, then centrifuged, washed, and dried in a vacuum oven to obtain a white powder, namely 3,5-N-methylbenzamide-2,4-dihydroxybenzophenone. The calculated yield according to Equation 1 was 87%.
[0032] Formula 1
[0033] Wherein, m2 is the mass of the product actually obtained, n1 is the amount of 2,4-dihydroxybenzophenone, and M2 is the molar molecular weight of the ...
Embodiment 2
[0049] Example 2 Synthesis of 3,5-di-N-methylbenzamide-2,4-dihydroxybenzophenone in acetone---aluminum chloride system:
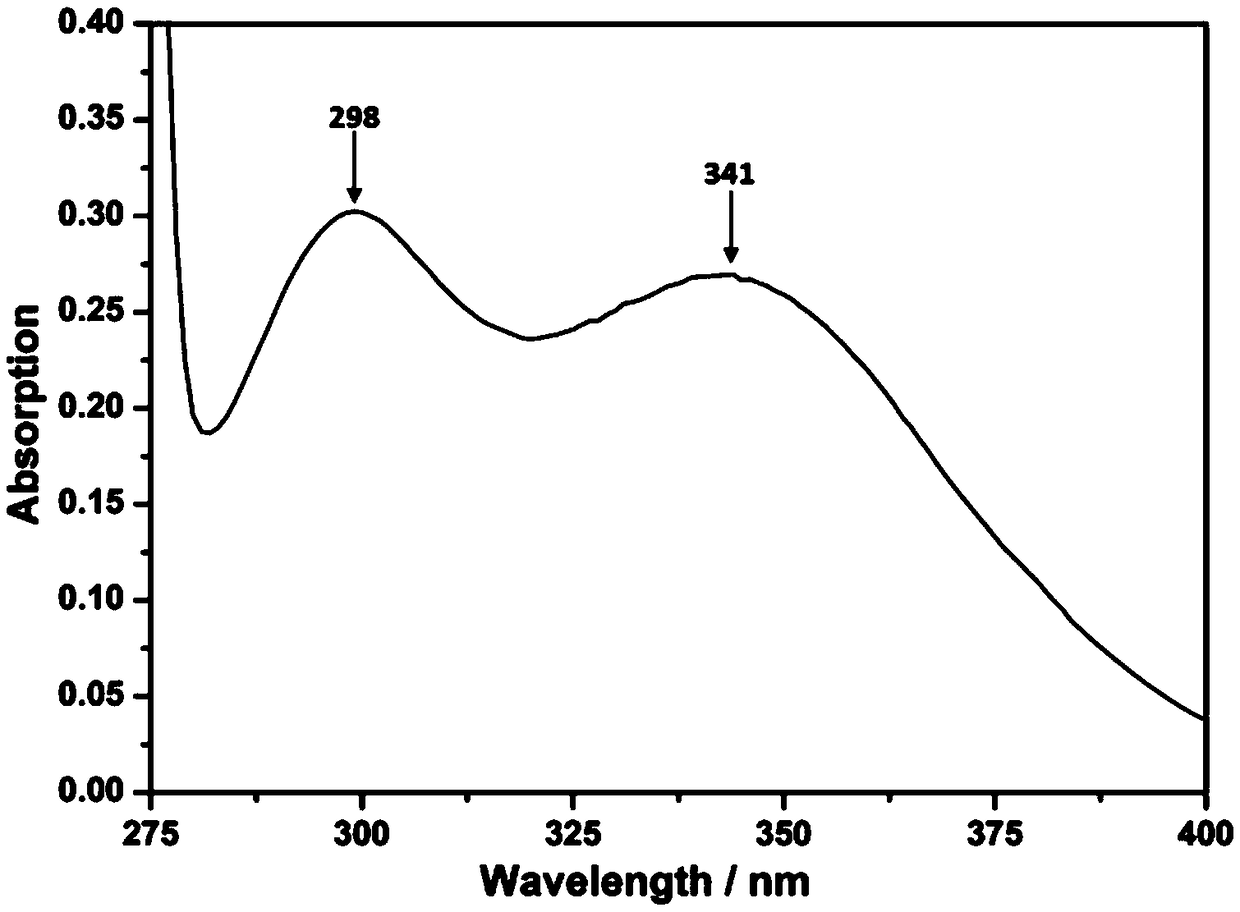
[0050]Add 21.4g (0.1mol) of UV-O, 20.2g (0.1mol) of N-methylolbenzamide and 150mL of acetone into a 250mL three-necked flask equipped with a stirring device, condenser and thermometer, and heat in an oil bath at 35°C Stir, add 6g of anhydrous aluminum trichloride as a catalyst, after 3 days, the reaction ends, after cooling to room temperature, solid-liquid separation is carried out, and the obtained solid is repeatedly washed with distilled water to neutrality, recrystallized with absolute ethanol, centrifuged again, washed, Dry in a vacuum oven to obtain a white powder, namely 3,5-bis-N-methylbenzamide-2,4-dihydroxybenzophenone. The yield calculated according to Equation 1 was 85%. The characterization method of the product is the same as in Example 1.
Embodiment 3
[0051] Example 3 Synthesis of 3,5-di-N-methylbenzamide-2,4-dihydroxybenzophenone in ethanol---strongly acidic ion exchange resin system:
[0052] Add 21.4g (0.1mol) of 2,4-dihydroxybenzophenone, 20.2g (0.1mol) of N-methylolbenzamide and 100ml of anhydrous Ethanol, heated and stirred in an oil bath at 35°C, then added 5g of strong acidic ion exchange resin as a catalyst, the reaction was completed after 3 days, solid-liquid separation was carried out after cooling to room temperature, and the obtained solid was repeatedly washed with distilled water to neutrality, then washed with absolute ethanol Recrystallized, then centrifuged, washed, and dried in a vacuum oven to obtain a white powder, namely 3,5-bis-N-methylbenzamide-2,4-dihydroxybenzophenone. The yield calculated according to Equation 1 was 80%. The characterization method of the product is the same as in Example 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap