A kind of preparation method and application of gelatin iron chelate
A technology of chelate and gelatin iron, which is applied in dyeing, textiles and papermaking, and can solve the problems of cotton having no affinity and being unable to dye cotton
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 25g of gelatin and 125g of distilled water into a four-neck reaction flask, raise the temperature to 40°C and stir for 20min. Add 1.1 g of ferrous sulfate heptahydrate and 5 g of distilled water into a beaker to dissolve, then pour into a separatory funnel. The material in the separatory funnel was added to the four-necked reaction flask at a rate of 1 drop per second. For the first time, use 30% sodium hydroxide solution to adjust the pH value of the reaction solution to 11, and react at 30°C for 1 hour; Adjust the pH value of the reaction solution to 11 with sodium hydroxide solution, react at 30°C for 1 hour, cool to room temperature, and discharge to obtain an orange-red solution of gelatin iron chelate.
[0036] (2) Preparation of bis(3-chloro-2-hydroxypropyl)tetramethylethylenediammonium dichloride: add 49g of 18% hydrochloric acid into a 250mL four-necked reaction flask, start stirring, and slowly dissolve the four Add 29 g of methylethylenediamine into...
Embodiment 2
[0045] (1) Preparation of gelatin iron chelate: with embodiment 1.
[0046] (2) Preparation of two (3-chloro-2-hydroxypropyl) tetramethyl ethylene diammonium dichloride: same as Example 1.
[0047] (3) Cationic modification of cotton cloth: same as Example 1.
[0048] (4) the orange red solution of 350g gelatin iron chelate that step (1) is made is dissolved in water and is made gelatin iron chelate solution and is settled to 1 liter, makes gelatin iron chelate treatment liquid, cationic cotton cloth two Dipping and padding (room temperature, 80% liquid retention rate), pre-baking (105°C, 5min), and baking (150°C, 3min) to obtain zwitterionic cotton cloth.
[0049] (5) Bath ratio 1:50, add water, amaranth 1% owf and unmodified cotton cloth or zwitterionic cotton cloth 3g, heat up to 80°C and keep it warm for 45min, wash in cold water, and dry at 80°C.
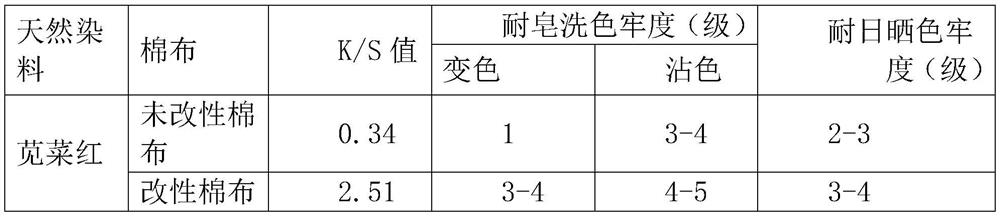
[0050] The test results are shown in Table 2.
[0051] Table 2 Unmodified cotton and modified cotton amaranth dyeing depth...
Embodiment 3
[0055] (1) Add 50 g of gelatin and 125 g of distilled water into a four-neck reaction flask, raise the temperature to 40° C. and stir for 20 min. Add 1.3 g of ferrous sulfate heptahydrate and 5 g of distilled water into a beaker to dissolve, then pour into a separatory funnel. The material in the separatory funnel was added to the four-necked reaction flask at a rate of 1 drop per second. For the first time, use 30% sodium hydroxide solution to adjust the pH value of the reaction solution to 11, and react at 30°C for 1 hour; Adjust the pH value of the reaction solution to 11 with sodium hydroxide solution, react at 30°C for 1 hour, cool to room temperature, and discharge to obtain an orange-red solution of gelatin iron chelate.
[0056] Other steps are with embodiment 1.
[0057] The test results are shown in Table 3.
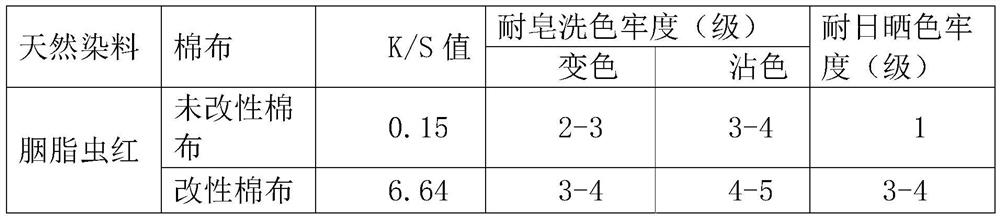
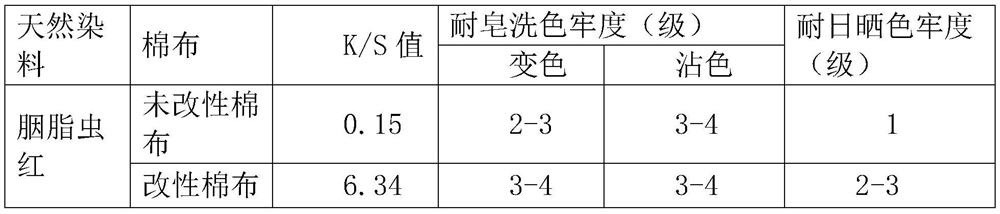
[0058] Table 3 Test results of cochineal red dyeing depth and fastness of unmodified cotton cloth and modified cotton cloth
[0059]
[0060] It can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com