α-(biphenoxy)acetate derivative and its synthesis method
A biphenoxy, synthetic method technology, applied in the field of α-(biphenoxy) acetate derivatives and their synthesis, to achieve the effects of mild reaction conditions, easy large-scale production, and accelerated reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Catalyst preparation: Sludge from a sewage treatment plant in Dalian, China, was dried in a muffle furnace at 105 °C to constant weight, and heated to 600°C, continuous carbonization for 4 hours, and the carbonaceous material (SW) derived from sewage sludge can be obtained after the temperature in the furnace drops to room temperature; in order to change the surface activity of SW, different acids are used to treat SW. During acid treatment, by mixing carbonized SW with the same volume of HCl (20.5 wt%), HClO 4 (35.4wt%), H 2 SO 4 (63.4wt%) and HNO 3 (40.5wt%) were impregnated for 24 hours to prepare 50 mL of different acid-treated SWs; then, wash SW-HCl and SW-HClO with deionized water, respectively 4 、SW-H 2 SO 4 and SW-HNO 3 Until the pH of the washing liquid reaches 6-7, the solid is recovered and dried at room temperature to obtain the corresponding acid-treated surface-modified sludge carbon catalyst SW-HCl, SW-HClO 4 、SW-H 2 SO 4 and SW-HNO 3 . Note: T...
Embodiment 1
[0038]
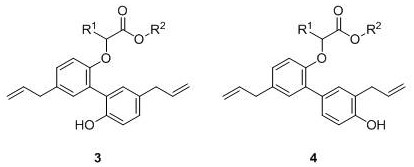
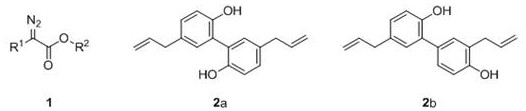
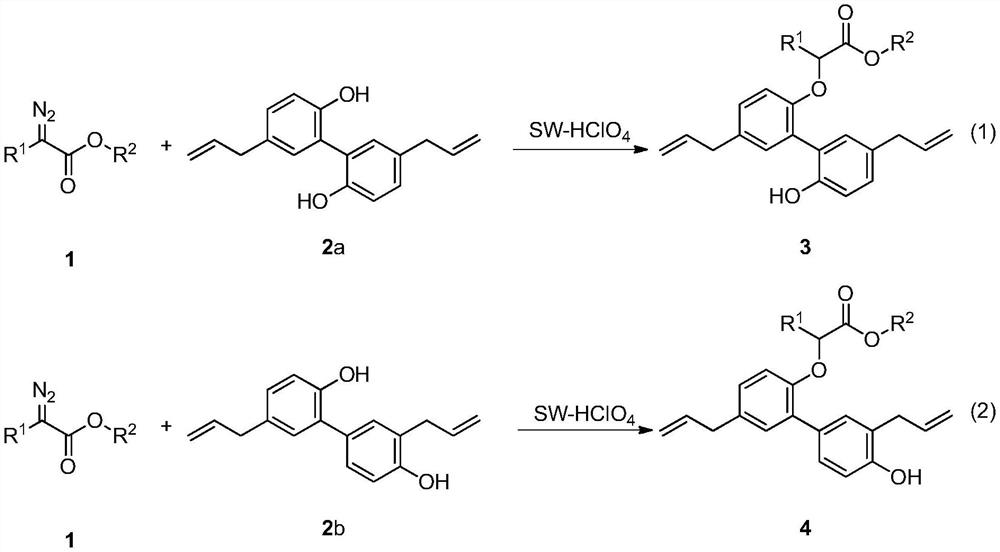
[0039] At room temperature, successively weigh methyl p-chlorophenyldiazoacetate 1a (0.5mmol), magnolol 2a (0.70mmol), SW-HClO 4 (50mg) into a dry and clean 25mL Schlenk reaction tube, under air atmosphere, add 5mL of 1,2-dichloroethane, stir at room temperature for 2-5 minutes, and transfer to an oil bath magnetic stirring heater at 70°C Reacted for 24 hours, TLC detected that the raw material had reacted completely, stopped the reaction, the reaction solution was cooled to room temperature, suction filtered, the filter cake was washed with dichloromethane until the filtrate was colorless, concentrated under reduced pressure to remove volatile components, and separated by silica gel column chromatography ( Eluent: petroleum ether (60-90° C.) / ethyl acetate, v / v=5:1), the target product 3a (168 mg, yield 75%) was obtained as a white solid, and the target product was tested by NMR and high Confirmation by resolution mass spectrometry.
Embodiment 2
[0040] Embodiment 2 (comparative example: different catalyst contrasts)
[0041]
[0042] At room temperature, successively weigh methyl-p-chlorophenyldiazoacetate 1a (0.5mmol), magnolol 2a (0.70mmol), SW-HCl (50mg) in a dry and clean 25mL Schlenk reaction tube, in air Under the atmosphere, add 5mL of 1,2-dichloroethane, stir at room temperature for 2-5 minutes, and transfer to an oil bath magnetic stirring heater at 70°C to react for 24 hours. TLC detects that the raw materials have reacted completely, stop the reaction, and The reaction solution was cooled to room temperature, filtered with suction, the filter cake was washed with dichloromethane until the filtrate was colorless, concentrated under reduced pressure to remove volatile components, separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate ester, v / v=5:1), the target product 3a (96 mg, yield 43%) was obtained as a white solid, and the target product was confirmed by NMR an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com