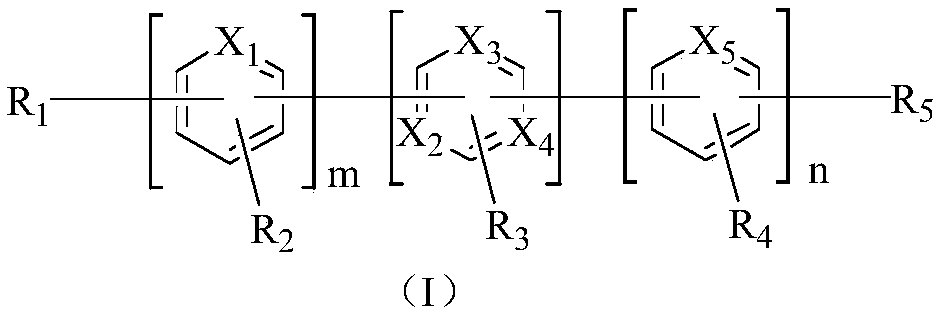Nitrogen heterocyclic compound and organic light-emitting display device
A nitrogen heterocyclic compound and a six-membered heterocyclic technology are applied in the field of organic electroluminescent materials, which can solve the problems of difficulty in developing doping materials, poor molecular thermal stability, low triplet energy level, etc. The effect of macromolecular density, luminous efficiency and lifetime improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
[0070] In a 250ml round bottom flask, add 2,6-difluoropyridineboronic acid (0.01mol), 3,6-dibromopyrazine (0.012mol) and tetrakis(triphenylphosphine)palladium (0.0006mol) into 15ml tetrahydrofuran THF , add 10ml 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified through a silica gel column to obtain an intermediate product M2.
[0071] In a 250ml round bottom flask, add intermediate product M2 (0.01mol), dimethylacridine (0.035mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and heated to reflux at 150°C for...
Embodiment 2
[0074]
[0075] In a 250ml round bottom flask, add 2,6-difluoropyridineboronic acid (0.02mol), 3,6-dibromopyrazine (0.012mol) and tetrakis(triphenylphosphine)palladium (0.0006mol) into 15ml tetrahydrofuran THF , add 10ml 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified by a silica gel column to obtain an intermediate product M3.
[0076] In a 250ml round bottom flask, add intermediate product M3 (0.01mol), diphenylamine (0.045mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and heated to reflux at 150°C for 12h. A...
Embodiment 3
[0079]
[0080]
[0081] In a 250ml round bottom flask, 2-phenyl, 6-fluoropyridineboronic acid (0.02mol), 3,6-dibromo, 5-phenylpyrazine (0.012mol) and tetrakis (triphenylphosphine) palladium ( 0.0006mol) into 15ml tetrahydrofuran THF, add 10ml 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified through a silica gel column to obtain an intermediate product M5.
[0082] In a 250ml round bottom flask, add intermediate product M5 (0.01mol), dimethylacridine (0.025mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com