Interferon recombinant fusion protein and application thereof
A technology of fusion protein and interferon, which is applied in the direction of interferon, fusion with toxin, cytokine/lymphokine/interferon, etc., which can solve problems such as interference with antiviral function, chronic virus infection, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of CaIFNα-linker-RTB target protein
[0035] 1.1 Acquisition of CaIFNα-linker-RTB gene
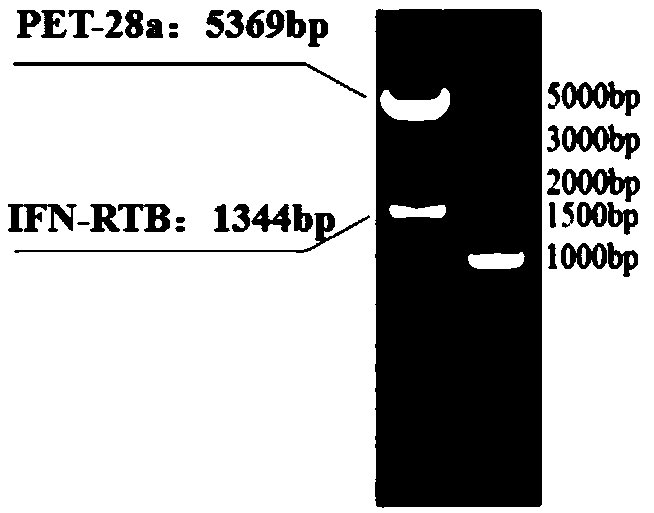
[0036] Synthesize the CaIFNα-linker-RTB target gene, add canine interferon α downstream (G 4 S) 3 Linker sequence, add ricin B chain sequence, and add NdeI and XhoI restriction enzyme sites at both ends of the target gene. The synthesized target gene nucleotide sequence was connected to the pUC57 vector, and the plasmid was named pUC57-CaIFNα / RTB.
[0037] The synthesized pUC57-CaIFNα / RTB was identified by double enzyme digestion and sequencing to ensure the correct sequence of the target gene.
[0038] 1.2 Construction of Escherichia coli recombinant expression vector
[0039] After the target fragment was identified correctly by sequencing and double enzyme digestion, the pUC57-CaIFNα / RTB plasmid and PET28a sequence were double digested with NdeI and XhoI, and the CaIFNα / RTB target fragment was connected to the PET28a vector fragment by DNA gel recovery a...
Embodiment 2
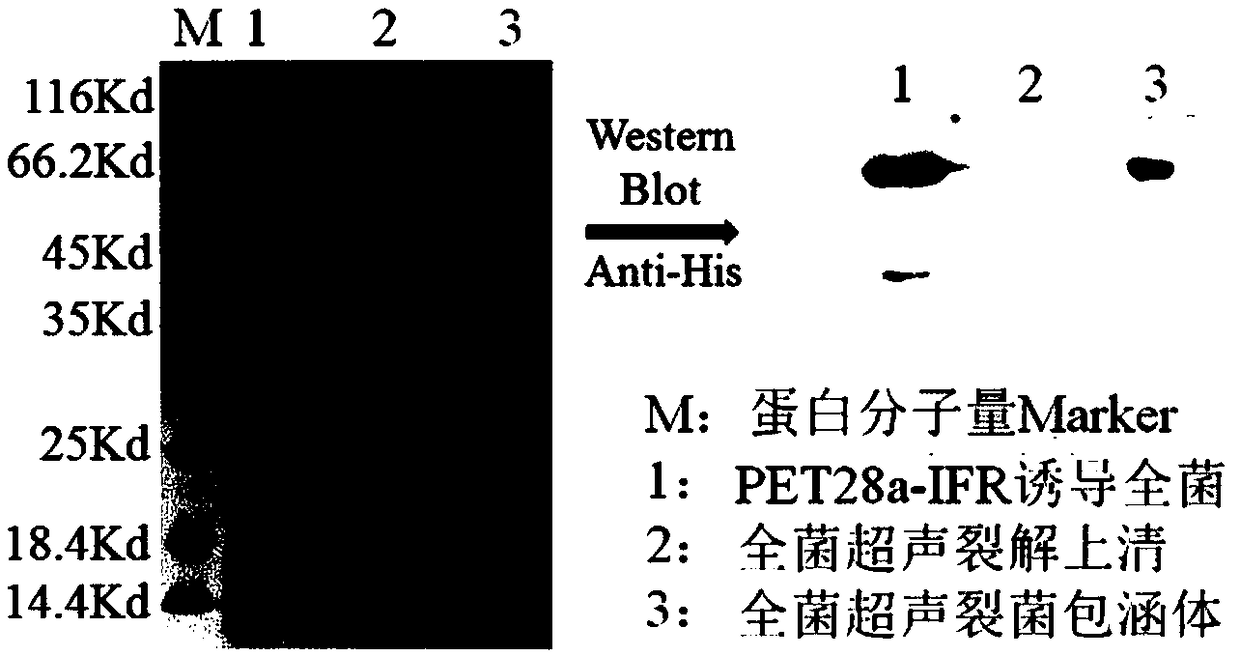
[0049] Example 2 Activity detection of CaIFNα-linker-RTB fusion protein
[0050] 2.1 Cytotoxicity of CaIFNα / RTB fusion protein
[0051] Using the MTT method to detect the toxic effect of the recombinant CaIFNα / RTB fusion protein obtained in the experiment on MDCK cells, the main steps are as follows:
[0052] (1) Digest well-grown MDCK cells with trypsin, centrifuge and resuspend to make a cell suspension, count on a counting plate, and dilute with 1×10 5 cells / mL to inoculate a 96-well plate, 100 μL per well. 37°C, 5% CO 2 Incubate overnight in an incubator to allow the cells to adhere to a monolayer.
[0053] (2) The CaIFNα / RTB fusion protein obtained in the experiment, according to 10 2 、10 1 、10 0 、10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 μg / mL for dilution.
[0054] (3) Take out the pre-spread 96-well culture plate, add the diluted protein sample into the corresponding well, 100 μL per well, make 3 duplicate wells for each concentration, 37°C, 5% CO 2 Cultiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com