Reagent for fast colorimetric detection on nitrate radicals and nitrite radicals
A technology of nitrite and nitrate, which is applied to measurement devices, material analysis, instruments and other directions by observing the influence on chemical indicators, can solve the problems of low sensitivity, long reaction time, complex probe molecule synthesis, etc. Strong anti-interference, short reaction time, real-time colorimetric detection of nitrate and nitrite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
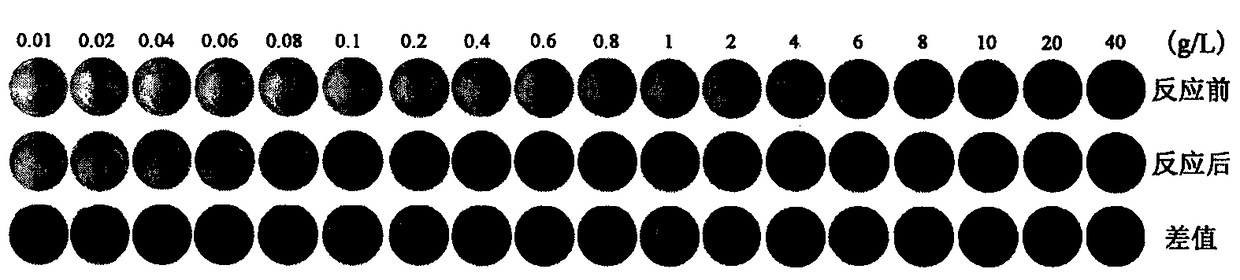
[0036] At room temperature, weigh 0.1mg, 0.2mg, 0.4mg, 0.6mg, 0.8mg, 1mg, 2mg, 4mg, 6mg, 8mg, 10mg, 40mg, 70mg, 100mg and 400mg of N-benzoanilide respectively dissolved in In 10mL of perchloric acid with a concentration of 70%, stir evenly to obtain a reagent for rapid colorimetric detection of nitrate and nitrite;
[0037] Mix the prepared reagents of different concentrations with 1M KNO 3 Scanning pictures of the flat plate before and after the reaction are as follows: figure 1 shown by figure 1 It can be seen that: the reagent before the reaction gradually changes from colorless to deep yellow along with the increase of N-benzoanilide concentration, and the solution after the reaction gradually changes from light yellow to brownish yellow; in order to visually compare the reaction The change of the color before and after the reaction is shown in the third row of picture 1 by directly subtracting the color after the reaction from the color before the reaction. It can be se...
Embodiment 2
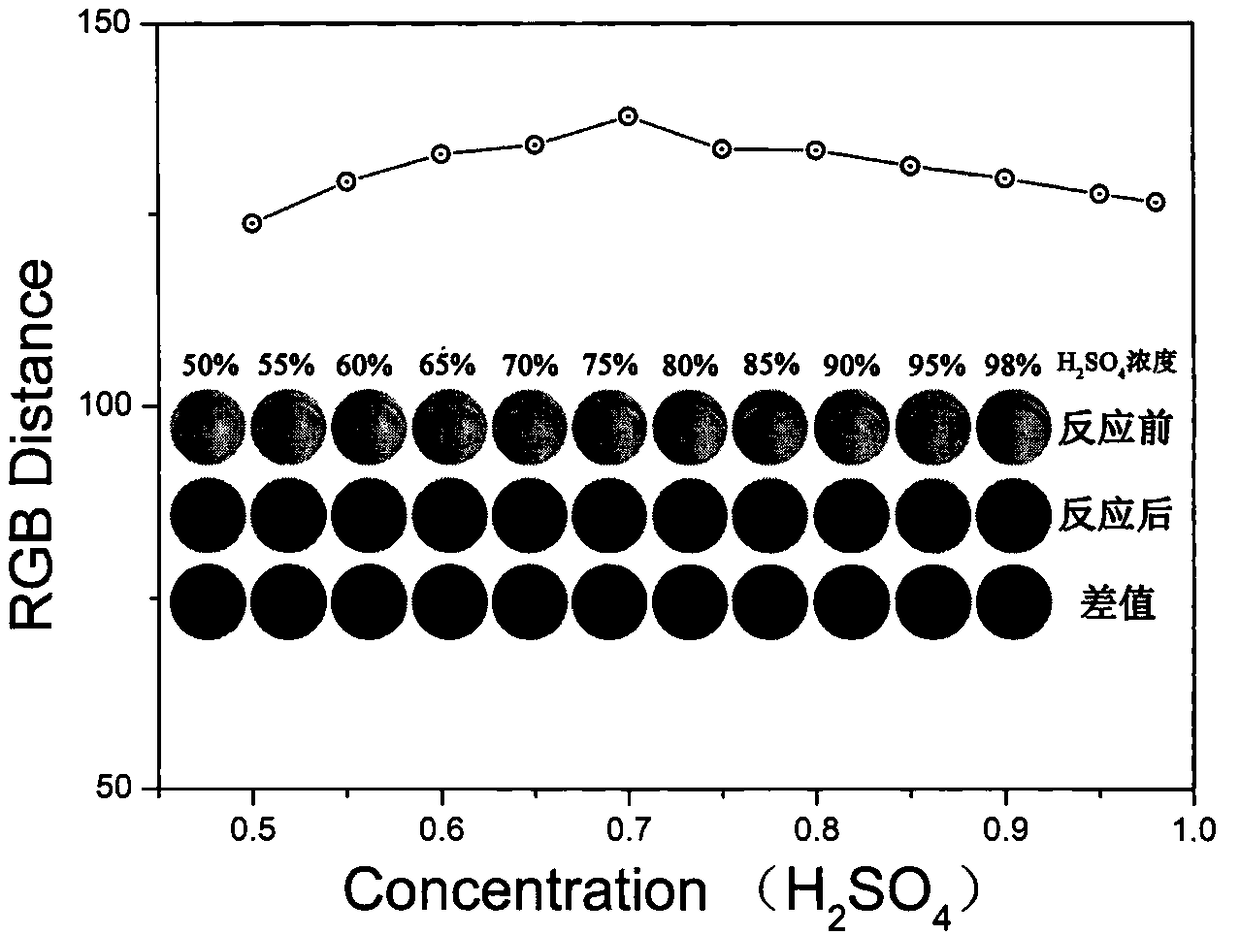
[0039] At room temperature, dilute 98% concentrated sulfuric acid with ultrapure water to 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90% and 95%, and then weigh 40mg of 3 -Amino-4-methoxyethoxy acetanilide was dissolved in 10mL of sulfuric acid of different concentrations and stirred evenly to obtain a reagent for rapid colorimetric detection of nitrate and nitrite;
[0040] Mix the prepared different sulfuric acid concentration reagents with 1M KNO 3 Scanning pictures of the flat plate before and after the reaction are as follows: image 3 As shown in (illustration), it can be seen that the reagent before the reaction gradually changes from colorless to light yellow with the increase of sulfuric acid concentration, and when the sulfuric acid concentration is 70%, the color reaches the darkest, and then gradually changes from colorless to light yellow with the increase of sulfuric acid concentration Pale yellow becomes colorless; And the solution after reaction gradually becomes...
Embodiment 3
[0042] At room temperature, dilute 57% hydriodic acid to 50% with ultrapure water, then weigh 1mg of 4,4'-diaminobenzanilide and dissolve it in 10mL of 50% hydriodic acid, stir evenly to obtain fast Reagents for colorimetric detection of nitrate and nitrite;
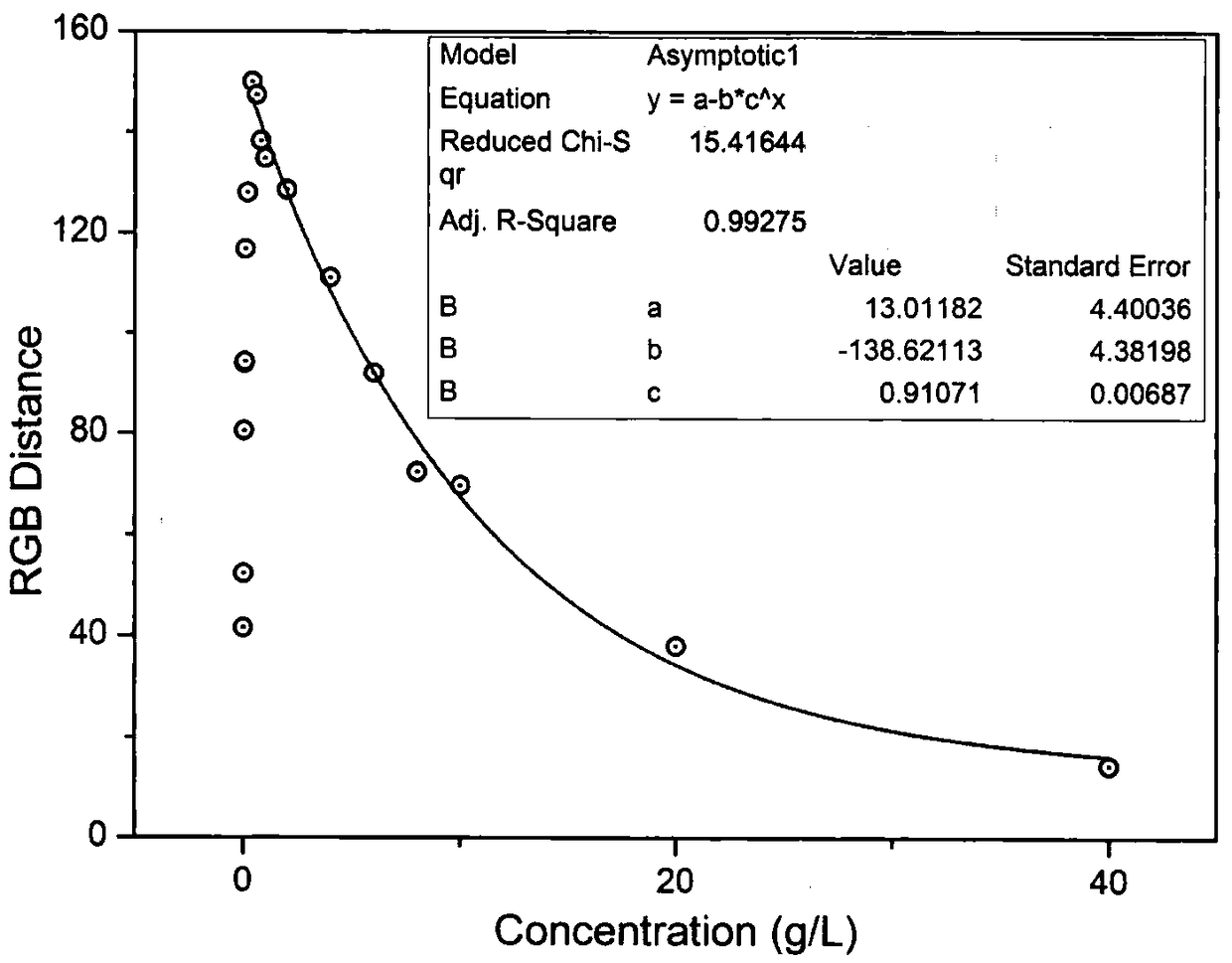
[0043] Mix the prepared detection reagent with 10mM, 9mM, 8mM, 7mM, 6mM, 5mM, 4mM, 3mM, 2mM, 1mM, 900μM, 800μM, 700μM, 600μM, 500μM, 400μM, 300μM, 200μM, 100μM, 90μM, 80μM, 70μM, 60 μM, 50 μM, 40 μM, 30 μM, 20 μM, 10 μM, 9 μM, 8 μM, 7 μM, 6 μM, 5 μM, 4 μM, 3 μM, 2 μM, 1 μM, 0.9 μM, 0.8 μM and 0 μM KNO 3 Ultrapure aqueous solution reaction, the color change before and after the reaction is obtained by scanning on a flat plate, such as Figure 4 shown by Figure 4 It can be seen that with KNO 3 As the concentration increases, the solution gradually changes from colorless to yellow, when KNO 3 The color change can be seen with the naked eye at a concentration of 10 μM; in order to compare the color change of the solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com