Methods and compositions for rna-guided treatment of HIV infection
A composition, HIV-1 technology, applied in retro RNA viruses, DNA/RNA fragments, gene therapy, etc., can solve problems such as inability to target and inhibit low-level viral genome expression and replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0127] The methods of the invention can be expressed in terms of the preparation of a medicament. Accordingly, the present invention includes the use of the agents and compositions described herein for the manufacture of a medicament. The compounds described herein are useful in therapeutic compositions and regimens or in the manufacture of medicaments for the treatment of diseases or conditions as described herein.
[0128] Any of the compositions described herein can be administered to any part of the host's body for subsequent delivery to target cells. Compositions can be delivered to, but are not limited to, the brain, cerebrospinal fluid, joints, nasal mucosa, blood, lungs, intestines, muscle tissue, skin, or peritoneal cavity of a mammal. In terms of route of delivery, the composition can be administered by intravenous, intracranial, intraperitoneal, intramuscular, subcutaneous, intramuscular, intrarectal, intravaginal, intrathecal, intratracheal, intradermal or transde...
example 1
[0152] Example 1: Materials and methods
[0153] Plasmid preparation : Various constructs, LTR-A, B, C and D, were generated using vectors containing human Cas9 and gRNA expression cassettes, pX260 and pX330 (Addgene).
[0154] Cell Culture and Stable Cell Lines : The TZM-b1 reporter and the U1 cell line were obtained from the NIH AIDS Reagent Program, and CHME5 microglial cells are known in the art.
[0155] Immunohistochemistry and western blot : A standard method for visualizing cells using immunocytochemistry and assessing protein expression by Western blotting.
[0156] Firefly-luciferase assay : According to the manufacturer's protocol, cells were lysed 24 hours after treatment using PassiveLysisBuffer (Promega) and analyzed with the Luciferase Reporter Gene Assay kit (Luciferase Reporter Gene Assay kit) ( Promega Corporation) for the determination. Luciferase activity was normalized to the number of cells determined by a parallel MTT assay (Vybrant, Invitrogen...
example 2
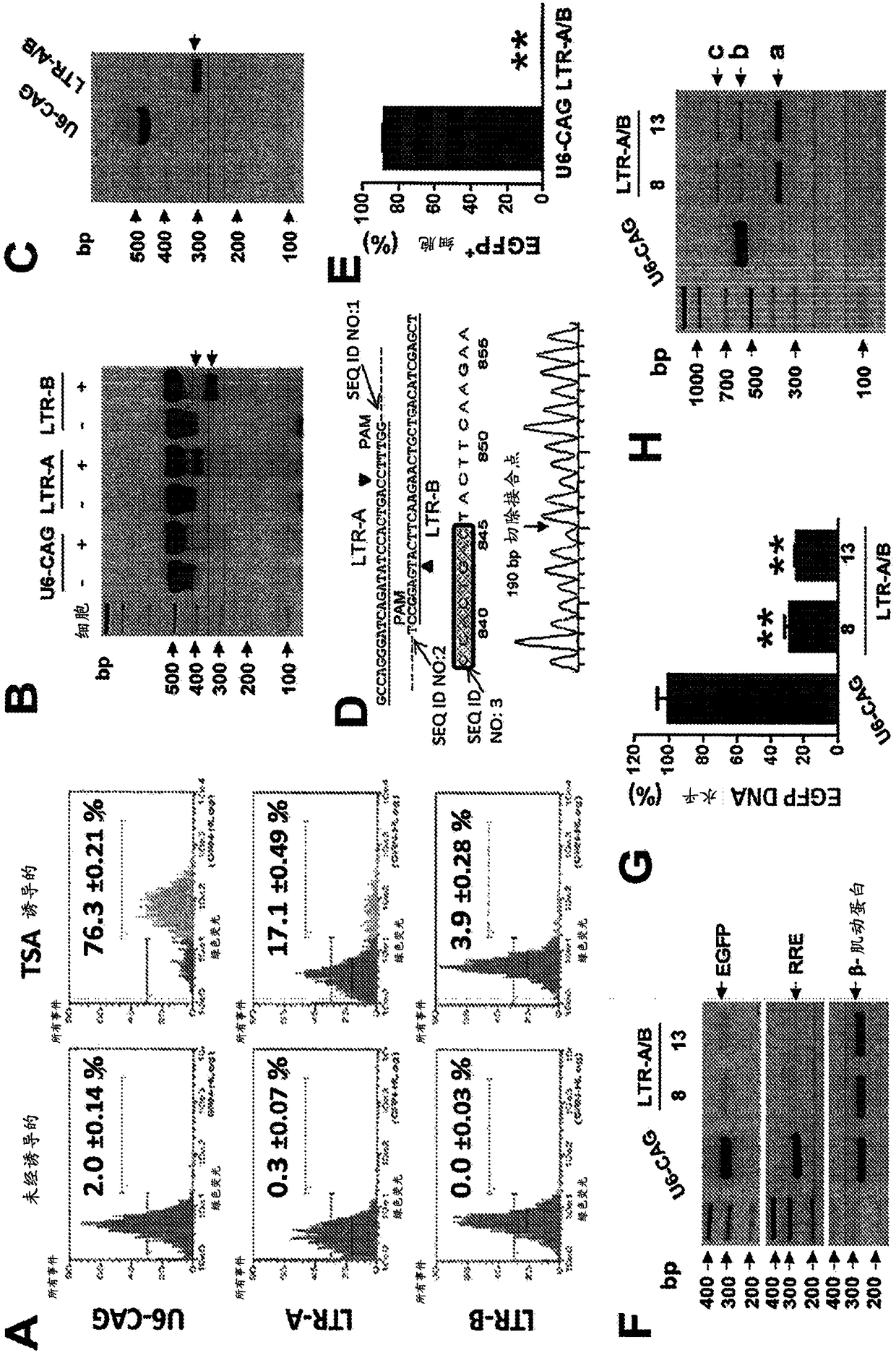
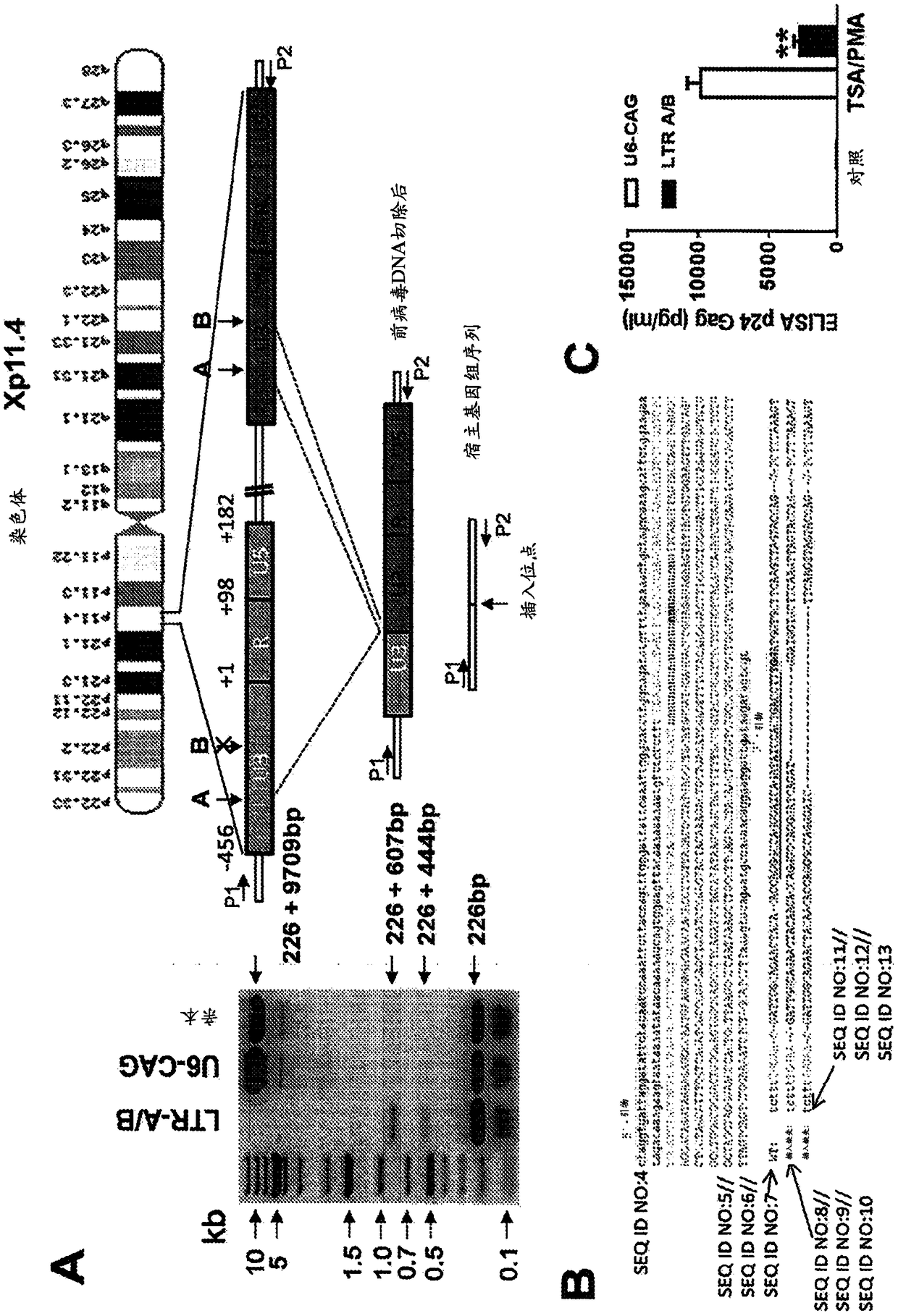
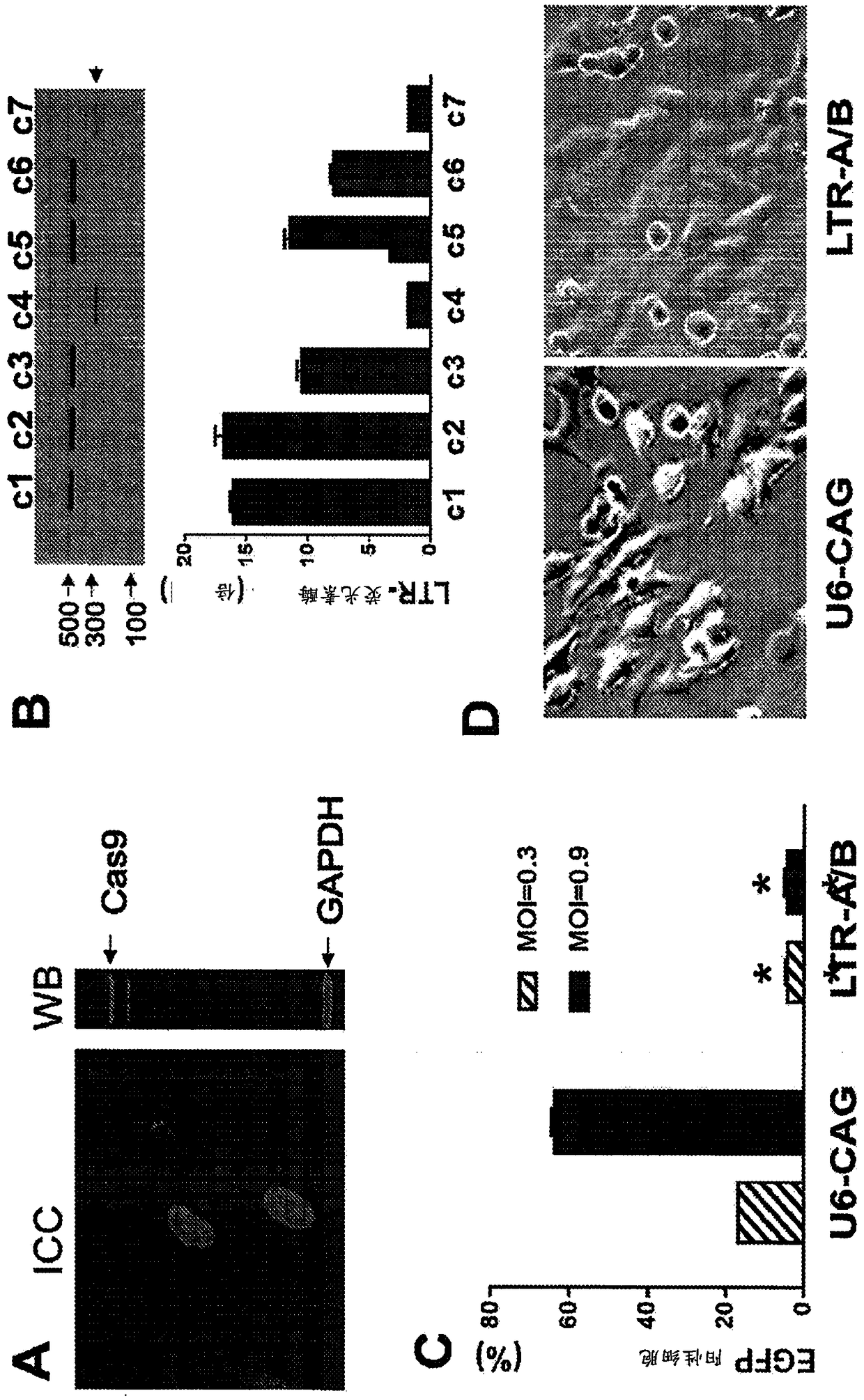
[0181] Example 2: Cas9 / LTR-gRNA inhibits HIV-1 reporter virus production in CHME5 microglial cells latently infected with HIV-1
[0182] We assessed the ability of HIV-1-directed guide RNA (gRNA) to abolish LTR transcriptional activity and eliminate proviral DNA from the genome of latently infected myeloid cells in the brain (a particularly refractory target population) as the HIV-1 reservoir. Our strategy focused on targeting the U3 region of the HIV-1 LTR promoter. Through bioinformatics screens and efficiency / off-target predictions, we identified four gRNA targets (protospacers; LTRA-D) that avoid conserved transcription factor binding sites, thereby minimizing the possibility of altering host gene expression ( Figure 5 and 13 ). We inserted DNA fragments complementary to gRNAs A-D into humanized Cas9 expression vectors (A / B in pX260; C / D in pX330) and tested their individual and combined ability to alter the activity of the integrated HIV-1 genome. We first utilized t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com