Application of a kind of ginsenoside grh2 in the preparation of anti-toxoplasma compound preparation and its medicine
A technology of ginsenoside and toxoplasma, which is applied in the field of medicine, can solve the problems of low efficiency and high toxicity of toxoplasma prevention and treatment drugs, and achieve the effect of inhibiting the proliferation of toxoplasma and prolonging the survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
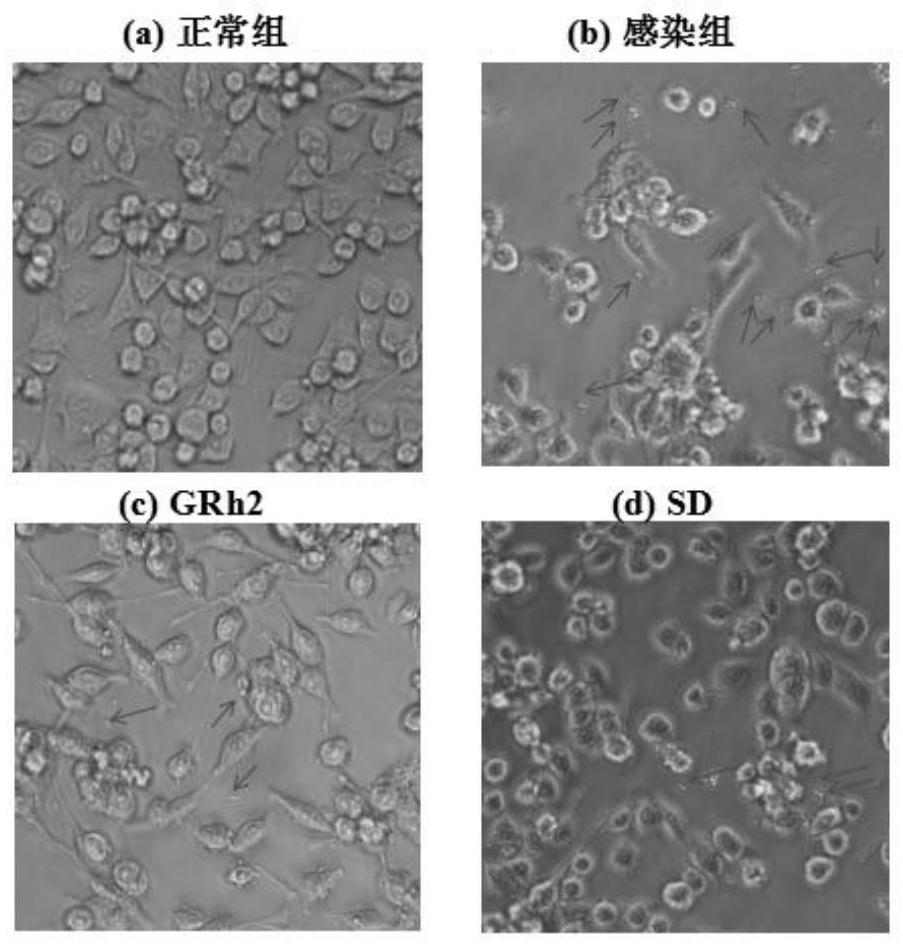
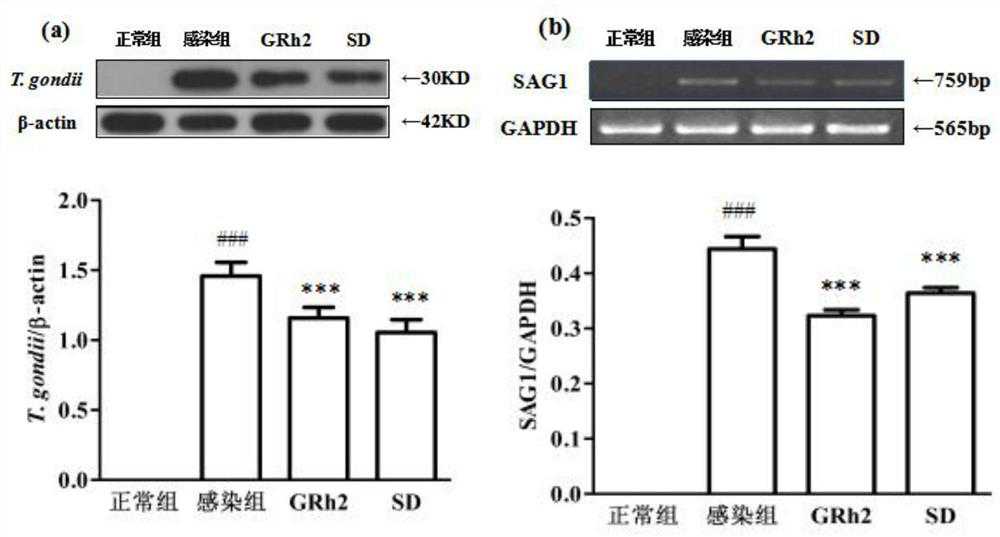
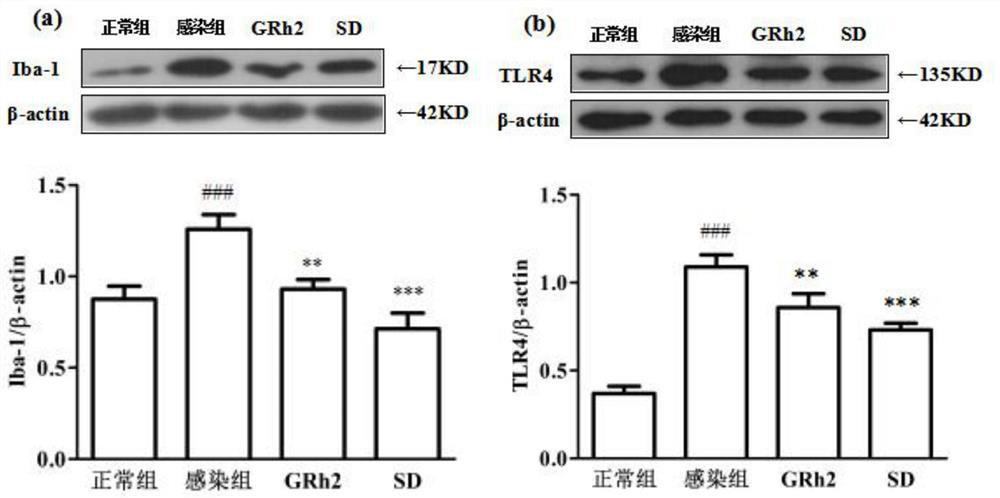
[0057] Embodiment 1 GRh2 resists Toxoplasma gondii infection and protects the host
[0058] 1 Experimental materials
[0059] 1.1 Experimental drugs
[0060] (S type) ginsenoside GRh2 (Shanghai Yuanye Biotechnology Co., Ltd., batch number: HA0408XB14, HPLC≥98%); (R type) ginsenoside GRh2 (Shanghai Yuanye Biotechnology Co., Ltd., batch number: Z14S63238, HPLC≥98%) ); positive control drugs: sulfadiazine (SD) (SIGMA-ALORICH, CAS: 68-35-9, 35033-100mg), sulfadiazine sodium (SD-Na) (Aladdin Industrial Corporation, CAS: 68-35-9, 35033-100mg).
[0061] 1.2 Experimental materials
[0062] Toxoplasma gondii: international standard virulent strain Toxoplasma gondii RH strain (ATCC); 50 SPF Balb / c female mice, weighing (20±2) g (Liaoning Changsheng Biotechnology Co., Ltd.); BV2 cells (ATCC) ;
[0063] DMEM medium (Gibco), fetal bovine serum FBS (Gibco); dimethyl sulfoxide DMSO (Sigma); MTT (Sigma); RIPA high-efficiency lysate (Solarbio); PMSF (Beiyuntian), BCA protein quantificatio...
Embodiment 2
[0149] The preparation of embodiment 2 GRh2 tablet
[0150] Raw materials and their dosage: 25g of GRh, 30g of mannitol, 2g of sodium carboxymethylcellulose and 1g of magnesium stearate.
[0151] Preparation process: drying each raw material, crushing and sieving through a 100-mesh sieve for pretreatment, mixing the main drug GRh2 with mannitol and sodium carboxymethylcellulose as auxiliary materials, adding an appropriate amount of 70% ethanol to make a soft material, passing through 14 mesh Sieve to form granules, blow dry at 55°C for 2 hours, sieve with 16 mesh and 100 mesh, add magnesium stearate and mix well, and press the mixture into tablets of appropriate size and weight to obtain GRh2 oral tablets.
Embodiment 3
[0152] Example 3 Preparation of GRh2 Injection
[0153] Raw material and dosage: GRh210g, PEG-4002.5L and normal saline for injection are added to 10L.
[0154] Preparation process: add GRh2 to the prescribed amount of PEG-400, stir to dissolve, add physiological saline for injection to make the final volume 10L and stir evenly. Sampling was taken to determine the pH value and content, and filtered through a 0.22 μm microporous membrane after passing the test. The GRh2 injection is obtained by aseptic potting.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com