Preparation method for ubenimex gamma crystal form
A technology of ubimethoxine and crystal form, which is applied in the field of gamma crystal form of ubenimex and its preparation, can solve the problems of long volatilization time, inability to meet the requirements of large-scale crystal form preparation, etc., and achieves high production cost and production conditions. Controllable, high-quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of Ubenimex gamma crystal form
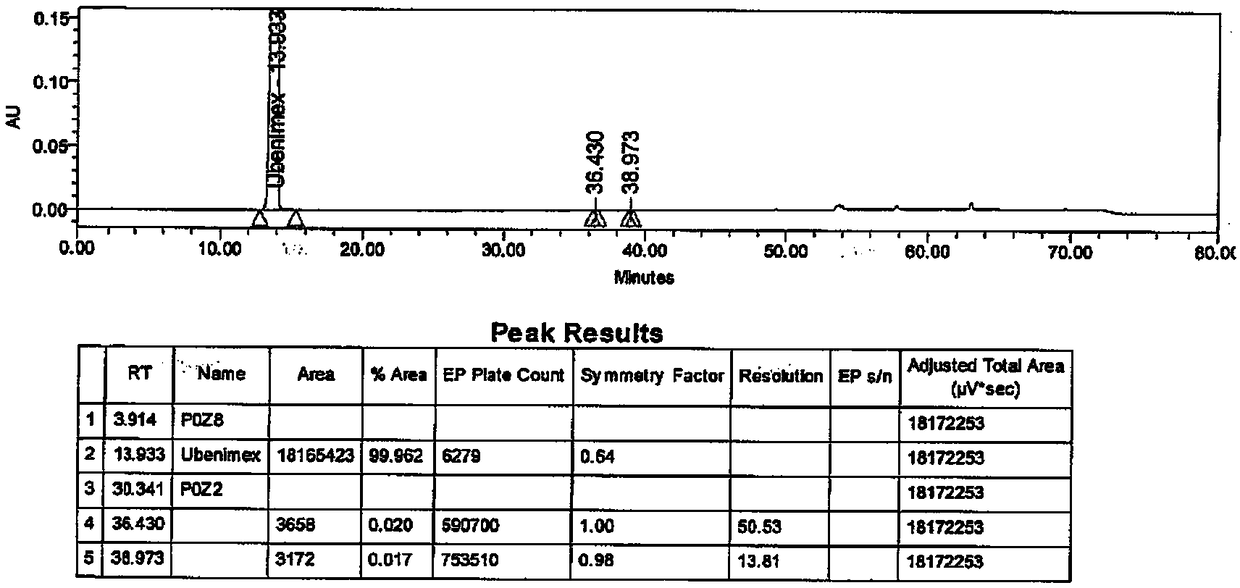
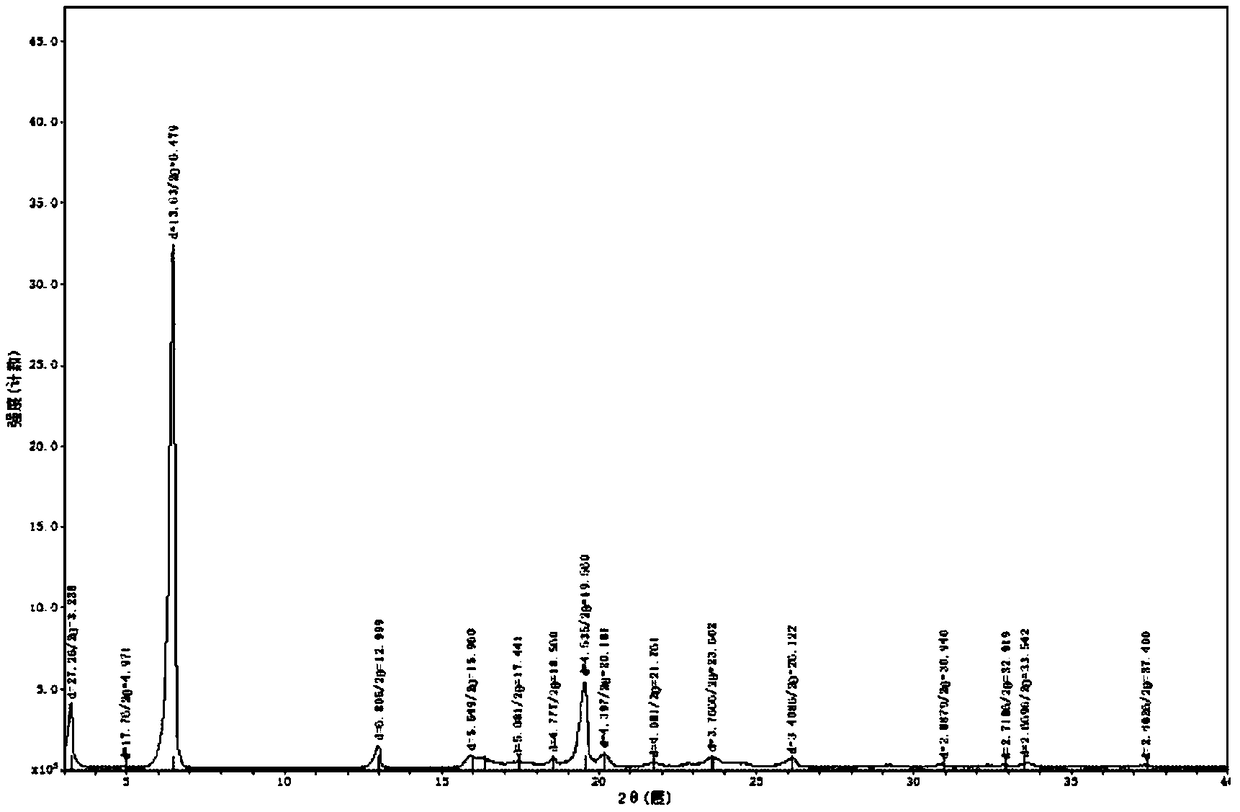
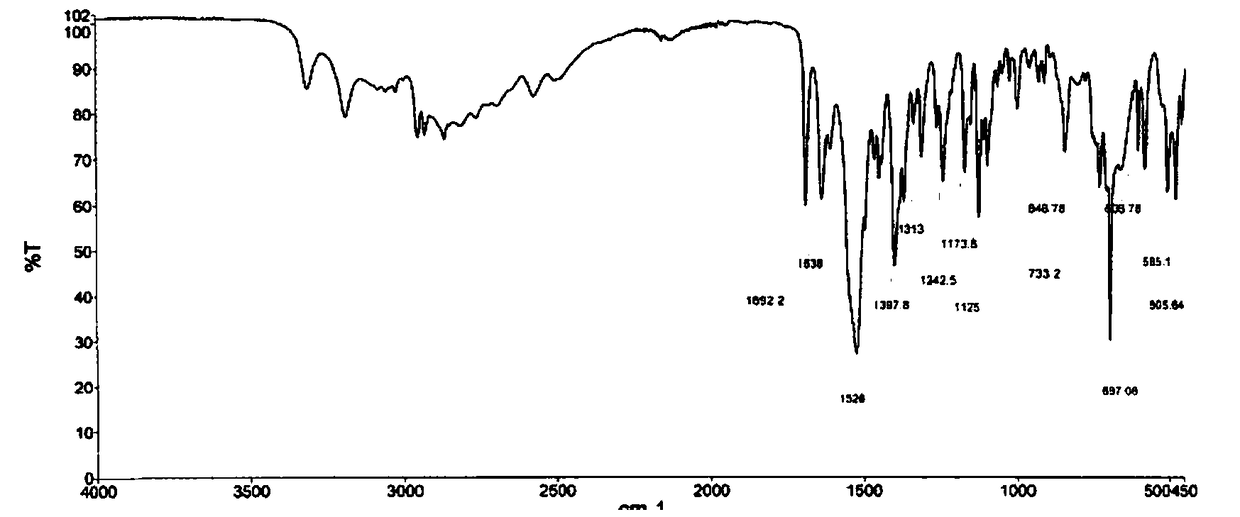
[0044] Disperse 19.40g of Ubenimex in 195ml of purified water, stir and beat at 45°C for 3h at a stirring rate of 360r / min, cool down in an ice bath to 5°C, stir and crystallize for 1h; filter under negative pressure, and place the filter cake in a vacuum at 70°C After drying in an oven for 8 hours, 17.90 g of white solid powder was obtained, with a yield of 92.3%. The HPLC purity is 99.96%, with a maximum of 0.02%. Using Cu-ka rays for X-ray powder determination, the spectrum has diffraction angles and interplanar spacings as shown in the table below:
[0045]
[0046]
[0047] The NMR results are as follows:
[0048] 1 H NMR (600MHz, DMSO): δ8.08(d,1H, J=7.8Hz), 7.32(m,5H), 3.96(q,1H, J=7.2Hz), 3.87(d,1H, J=3.0 Hz), 3.54(m, 1H), 2.96(m, 2H), 1.61(d, 2H, J=4.2Hz), 0.86(m, 6H)
[0049] 13C NMR (600MHz, DMSO): δ175.52(s, 1C), 172.09(s, 1C), 137.91(s, 1C), 129.92(s, 2C), 128.84(s, 2C), 126.94(s, 1C ),69....
Embodiment 2
[0051] Example 2: Preparation of Ubenimex gamma crystal form
[0052] Disperse 10.0g of Ubenimex in 150ml of purified water, stir and beat at 40°C for 16h, stirring at a rate of 500r / min, cool down in an ice bath to 10°C, stir and crystallize for 1h; filter under negative pressure, and place the filter cake in a vacuum at 50°C After drying in an oven for 16 hours, 9.07 g of white solid powder was obtained, with a yield of 90.07%. The HPLC purity is 99.94%, the maximum single impurity is 0.03%, and the total impurity is 0.06%.
[0053] Its NMR data and powder diffraction data are basically consistent with the data of Example 1.
Embodiment 3
[0054] Example 3: Preparation of Ubenimex gamma crystal form
[0055] Disperse 10.0g of Ubenimex in 50ml of purified water, stir and beat at 70°C for 2h, stirring at a rate of 200r / min, cool down in an ice bath to 10°C, stir and crystallize for 0.5h; filter under negative pressure, and place the filter cake at 60°C Dry in a vacuum oven for 24 hours to obtain 9.07 g of white solid powder with a yield of 90.07%. The HPLC purity is 99.94%, the maximum single impurity is 0.03%, and the total impurity is 0.06%.
[0056] Its NMR data and powder diffraction data are basically consistent with the data of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com