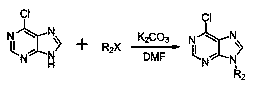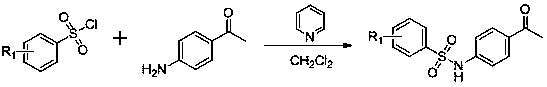Purine-ring-containing benzene sulfonamide chalcone derivative and preparation and application methods thereof
A technology of chalcones and benzenesulfonamides, which is applied to the chalcone derivatives of benzenesulfonamide chalcones containing purine rings, their preparation and application, and achieves the effects of low production cost, simple production process, and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
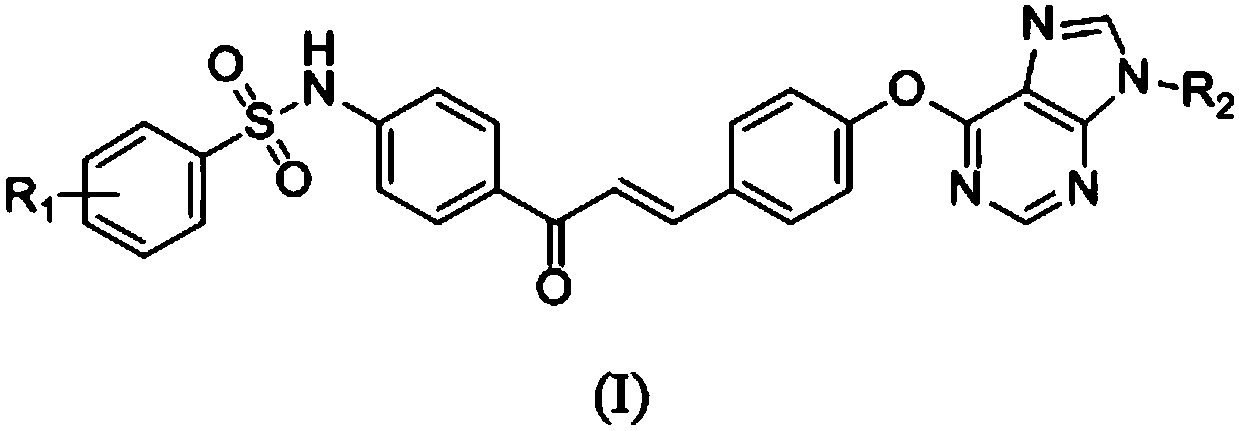
[0064] Example 1: (E)-1-(4-((4-methylphenyl)sulfonamido)phenyl)-3-(4-((9-methyl-9H-purin-6-yl) Synthesis of oxy)phenyl)-2-propenyl ketone (compound number A 1 )
[0065] (1) Preparation of N-(4-acetylphenyl)-4-methylbenzenesulfonamide
[0066] Add p-aminoacetophenone (2.00g, 14.80mmol) into a 50mL single-necked flask and add 10mL of dichloromethane, then add pyridine (1.17g, 14.80mmol) to the system, stir at room temperature for 1 hour, the reaction system is orange To the yellow liquid, p-toluenesulfonyl chloride (3.10g, 16.28mmol) was slowly added dropwise, the color of the system deepened, and finally turned into a red clear liquid. The reaction was tracked by thin-layer chromatography, and the reaction was basically complete after 10 hours of reaction. After the reaction, the reaction system was poured into a beaker, washed with water, poured off the water layer, washed repeatedly 5 times, and finally transferred the organic layer containing a small amount of water into ...
Embodiment 2
[0073] Example 2: (E)-1-(4-((4-methylphenyl)sulfonamido)phenyl)-3-(4-((9-ethyl-9H-purin-6-yl) Synthesis of oxy)phenyl)-2-propenyl ketone (compound number A 2 )
[0074] Steps (1)-(2) are the same as in Example 1;
[0075] (3) Preparation of 6-chloro-9-ethyl-9H-purine
[0076] 6-chloropurine (2.00g, 12.94mmol) was added to a 25mL single-necked flask, and 8mL of DMF was added, stirred at room temperature, the system was a yellow liquid, potassium carbonate (3.58g, 25.88mmol) was added to the system, and Stir for half an hour, then slowly add bromoethane (4.23g, 38.82mmol) dropwise to the system, and the reaction is basically complete after 24 hours. After the reaction ends, the reaction system is poured into a beaker, washed with saturated ammonium chloride solution, Then extract with dichloromethane, wash repeatedly 3 times, take the organic layer, dry with anhydrous magnesium sulfate for half an hour, filter, remove the magnesium sulfate solid, rotary steam, remove DMF, obt...
Embodiment 3
[0079] Example 3: (E)-1-(4-((4-methylphenyl)sulfonamido)phenyl)-3-(4-((9-benzyl-9H-purin-6-yl) Synthesis of oxy)phenyl)-2-propenyl ketone (compound number A 3 )
[0080] Steps (1)-(2) are the same as in Example 1;
[0081] (3) Preparation of 6-chloro-9-benzyl-9H-purine
[0082] 6-chloropurine (2.00g, 12.94mmol) was added to a 25mL single-necked flask, and 8mL of DMF was added, stirred at room temperature, the system was a yellow liquid, potassium carbonate (3.58g, 25.88mmol) was added to the system, and Stir for half an hour, and slowly add benzyl chloride (3.28g, 25.88mmol) dropwise to the system. After 24 hours, the reaction is basically complete. After the reaction, the reaction system is poured into a beaker, washed with saturated ammonium chloride solution, and then Extract with dichloromethane, wash repeatedly 3 times, take the organic layer, dry with anhydrous magnesium sulfate for half an hour, filter, remove the magnesium sulfate solid, rotary steam, remove DMF, ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com