Preparation method of cyclodextrin terminated star-shaped polymer
A star-shaped polymer and cyclodextrin technology, which is applied in the field of star-shaped polymer preparation, can solve problems such as lack, and achieve the effects of expanding types, expanding application range, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of six-arm star polymer (i)
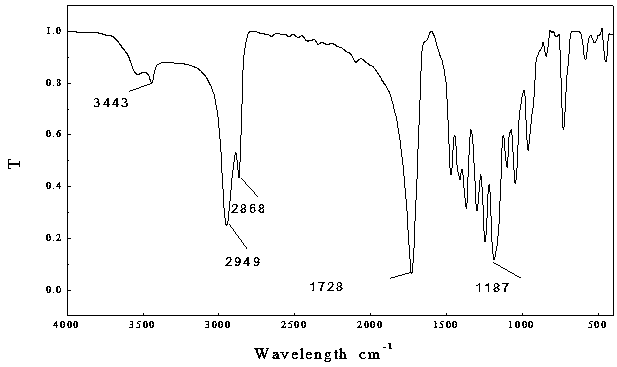
[0023] Under anhydrous and oxygen-free operation, weigh 0.2543 g (1 mmol) of dipentaerythritol into a 50 mL eggplant-shaped flask, then add ε-caprolactone 13.30 mL, stir magnetically for 10 minutes, and then add the catalyst containing stannous isooctanoate 0.1 mL of toluene solution 20 mL, and stirred until the solid was completely dissolved; the reaction was gradually heated to 100 ° C, and continued to react at this temperature for 24 h; after the reaction was completed, the reaction solution was cooled to room temperature, and then the mixture was dripped A white solid was precipitated in methanol. After filtration, the filter cake was washed three times with methanol and water, and then dried under vacuum at 40°C for 24 h to obtain the product (i) in the form of white powder.
[0024] Using infrared to analyze its structure, the results are shown in figure 1 , 3443 cm -1 It is the stretching vibration absorp...
Embodiment 2
[0025] Embodiment 2: the preparation of N-BOC-4-aminobenzoic acid
[0026] Weigh 1.00 g of p-aminobenzoic acid and add it to a 50 mL round bottom flask, then add 10 mL of dioxane and 10 mL of distilled water, stir at room temperature for 10 min; add triethylamine in an ice bath at 0°C to adjust the pH of the solution to 9 ~10; Add 3.4 mL of di-tert-butyl dicarbonate in portions within 1.5 h. After the dropwise addition, continue to stir for 2 h under ice bath, and then warm up to room temperature. After reacting for 24 h, the mixture was added dropwise to 100 mL of NaHSO4 solution with a mass fraction of 10%, a large amount of white precipitates were precipitated, the precipitates were collected and washed with water several times, and dried under vacuum at 120 °C to obtain white powdery N -BOC-4 aminobenzoic acid.
Embodiment 3
[0027] Example 3: Preparation of polymer (ii)
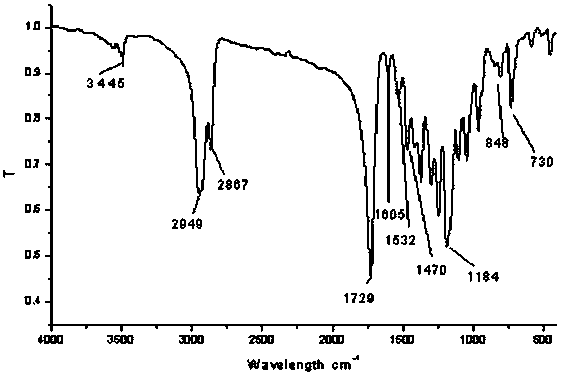
[0028] Weigh 1.00 g of six-armed star polymer (ⅰ) and 2 g of N-BOC-4-aminobenzoic acid into a 50 mL round bottom flask, add 30 mL of dichloromethane and stir at room temperature for 10 min; add 2.1742 g dicyclohexylcarbodiimide and 0.0515 g 4-dimethylaminopyridine, the solution gradually became turbid from clear; after continuing to react for 48-72 hours at room temperature, a large amount of white solid precipitated, filtered and poured the filtrate into a round bottom flask , Seal and freeze at -20°C for 2 hours, then filter again, continue to freeze the filtrate, repeat 3-5 times, until no precipitation occurs; concentrate the treated filtrate under reduced pressure until the mixture becomes milky, add methanol to soak, and white precipitates precipitate out, The filter cake was washed several times with methanol and water, respectively, and dried under vacuum at 40°C to obtain the product (ii) as a white solid powder.
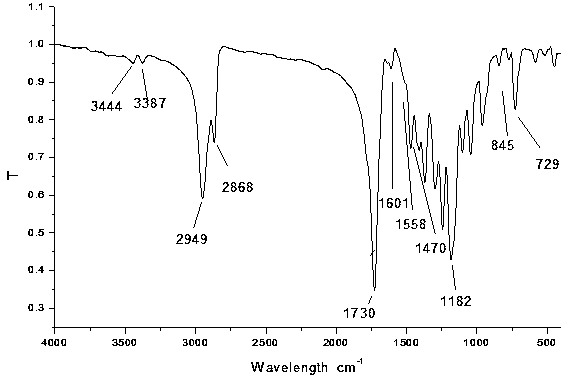
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com