Method for preparing cobalt chloride through evaporation and crystallization of cobalt chloride
An evaporation crystallization and cobalt chloride technology, which is applied in the preparation field of cobalt chloride, can solve the problems of long process flow, small cobalt chloride particles, and large equipment investment, and achieves the effect of stable evaporation process and excellent crystals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
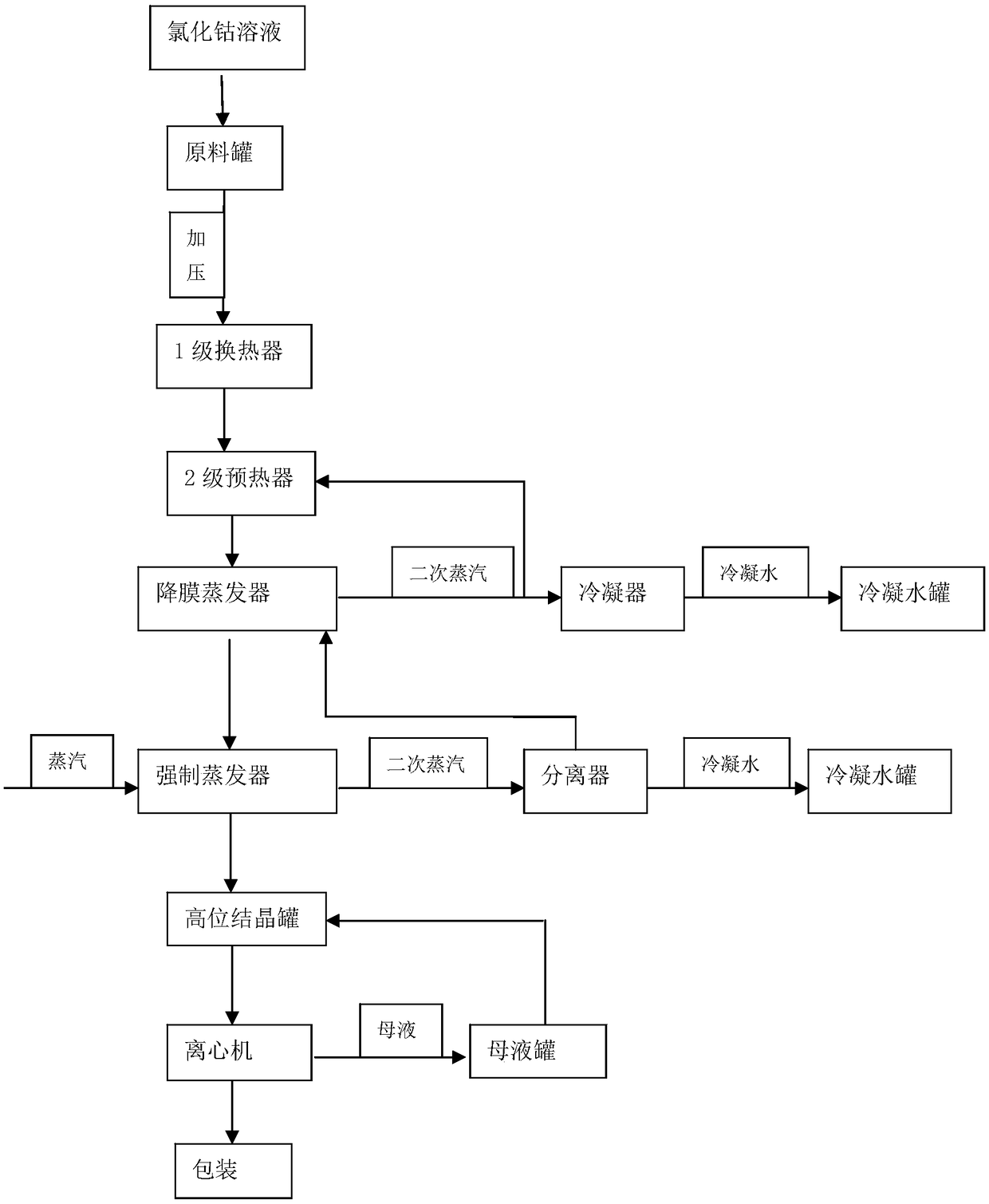
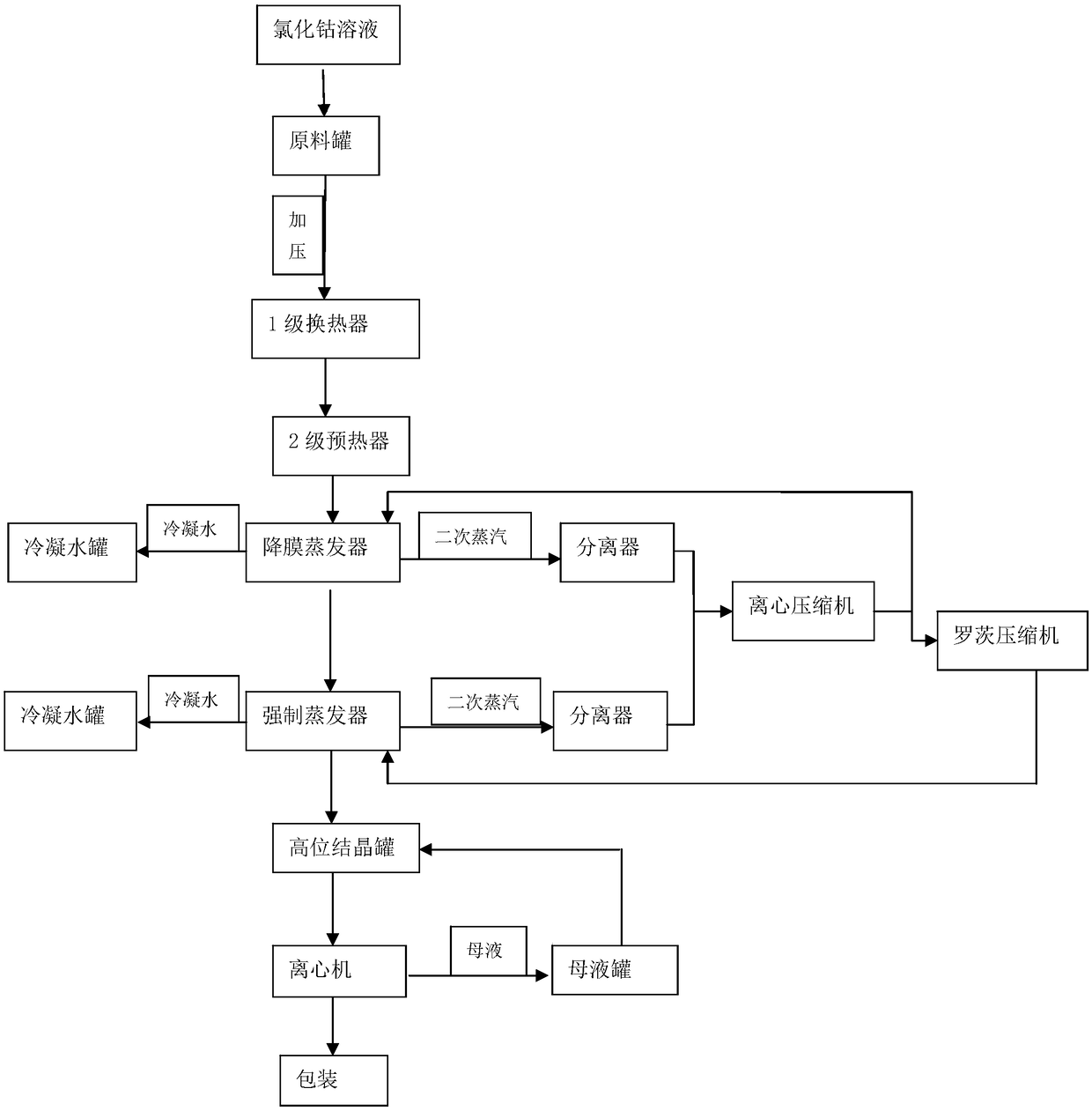
[0037] (1) Preheating:
[0038] Pump cobalt chloride solution with a temperature ≥ 10°C into the stock solution tank, pressurized by the raw material pump, enter the first-stage plate heat exchanger to preheat to 65°C, then heat to 77°C through the second-stage preheater, and then enter In the falling film evaporator;
[0039] (2) Evaporation and concentration:
[0040] Falling film evaporation: pump the cobalt chloride solution preheated in step (1) into the second-effect falling-film evaporator. In the falling film evaporator, the raw material in the falling film evaporator tube exchanges heat with the heating steam outside the tube to boil and evaporate the raw material, and the raw material is concentrated and then pumped into the forced evaporator;
[0041] Forced evaporation: Fresh steam is introduced into the shell of the heat exchanger of the forced evaporator to exchange heat between the raw material in the tube of the forced evaporator and the shell steam to evapor...
Embodiment 2
[0051] (1) Preheating:
[0052] Pump the cobalt chloride solution with a temperature ≥ 10°C into the raw solution tank, pressurized by the raw material pump, enter the first-stage plate heat exchanger to preheat to 48°C, and then heat it to 65°C through the second-stage preheater, and then enter In the falling film evaporator;
[0053] (2) Evaporation and concentration:
[0054] Falling film evaporation: pump the cobalt chloride solution preheated in step (1) into the MVR falling film evaporator, the raw material in the falling film evaporator tube exchanges heat with the heating steam outside the tube to make the raw material boil and evaporate, and the raw material is concentrated and then pumped into the forced evaporator;
[0055] Forced evaporation: Fresh steam is introduced into the shell of the heat exchanger of the forced evaporator to exchange heat between the raw material in the tube of the forced evaporator and the shell steam to evaporate the raw material and con...
Embodiment 3
[0065] (1) Preheating:
[0066] Pump the cobalt chloride solution with a temperature ≥ 10°C into the stock solution tank, pressurized by the raw material pump, enter the first-stage plate heat exchanger to preheat to 60°C, then heat to 75°C through the second-stage preheater, and then enter In the falling film evaporator;
[0067] (3) cooling crystallization
[0068] In the crystallization process, the super-concentrated cobalt chloride solution that removes free water is added to the cold saturated cobalt chloride solution, so that the supersaturation of cobalt chloride is effectively controlled, so that the production can be carried out, and the obtained particles are very Large crystals in which the mass of the cold saturated solution of cobalt chloride is 5 times the mass of the ultra-concentrated solution of cobalt chloride.
[0069] Other steps are with embodiment 1.
[0070] Feed solution: salt content about 34%, density 1.34g / mL, PH>1.5, feed rate: 11.123T / h, evapor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com