Method for preparing 3-methyl-2-buten-1-ol through transesterification reaction of isopentenyl acetate
A technology for the transesterification of prenyl acetate and prenol, which is applied in the field of transesterification of prenyl acetate to prepare prenyl alcohol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
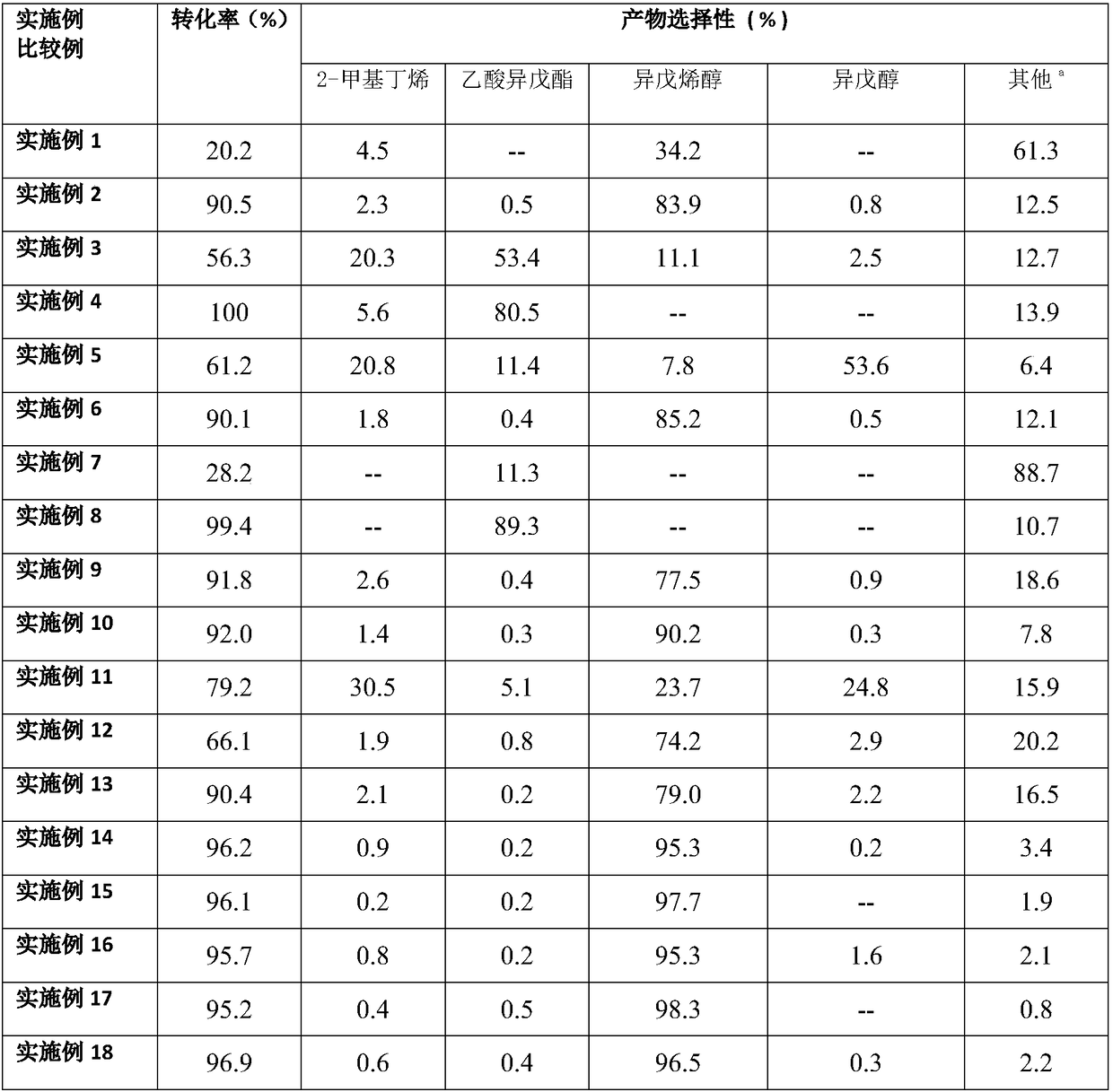
Embodiment 1
[0022] Embodiment 1 (comparative example)
[0023] The catalyst is CeO purchased from Sinopharm 2 , using a batch reactor, the catalytic reaction conditions are: 0.5g of prenyl acetate is mixed with 1.0mL of methanol, 50mg of catalyst is added, the reaction temperature is 140°C, the reaction pressure is 4Mpa, and the reaction time is 1.5h;
Embodiment 2
[0025] The catalyst is CeO 2 The carrier is loaded with precious metal Ir (Ir mass fraction is 2%). The specific preparation process is: impregnating the incipient wetness of a well-proportioned aqueous solution of chloroiridic acid onto the carrier for 24 hours, drying in an oven at 80°C for 12 hours, and drying in a muffle furnace. Calcined at 350°C for 2 hours to produce 2% Ir / CeO 2 solid acid catalyst. The prepared catalyst was reduced at 350° C. for 2 h under a hydrogen atmosphere. The reaction conditions are as follows: 0.5 g of prenyl acetate is mixed with 1.0 mL of methanol, 50 mg of catalyst is added, the reaction temperature is 140° C., the reaction pressure is 4 Mpa, and the reaction time is 1.5 h.
Embodiment 3
[0026] Embodiment 3 (comparative example)
[0027] Carrier CeO 2 Change to TiO 2 , other conditions are the same as in example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com