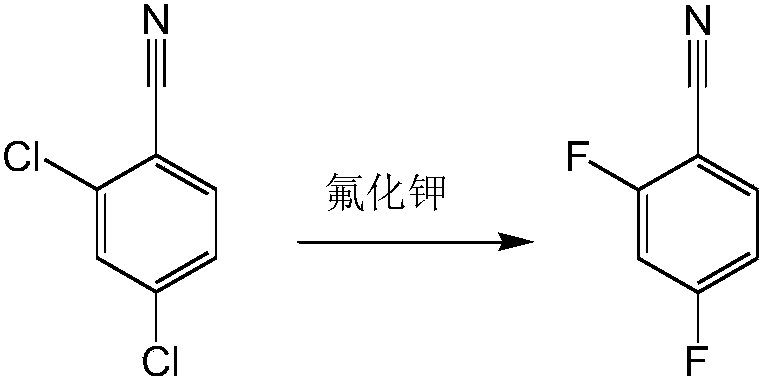
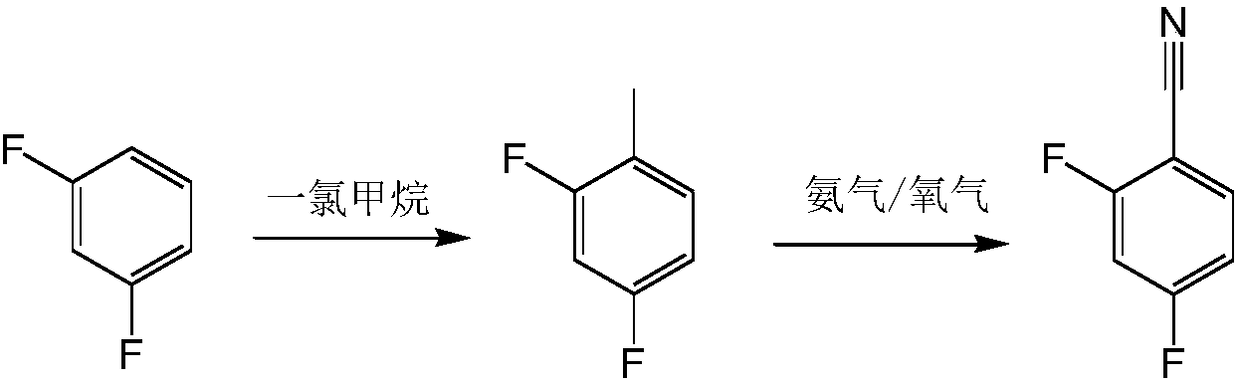
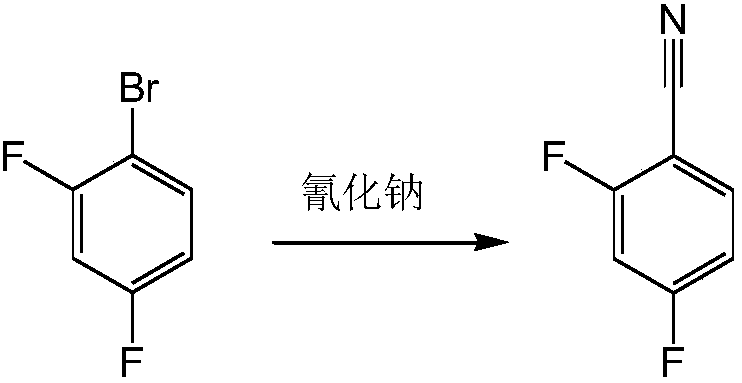
Preparation process for 2,4-difluorobenzonitrile
A technology that prepares and diofluorophenylpines can be used in the 2nd field. It can solve problems that are not suitable for industrial production, low product revenue, and high response temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of 2,4-difluorobenzonitrile
[0044] Add 314g (2.00mol) of 2,4-difluorobenzoic acid and 952g (8.00mol) of thionyl chloride to a 2000ml single-necked bottle, heat up and reflux for about 4 hours (the temperature of temperature rise and reflux is 78°C), and concentrate to dryness under reduced pressure , to obtain a yellow oil, that is, 2,4-difluorobenzoyl chloride, to obtain 318g (1.80mol), and the yield is 90%.
[0045] Add 310g (1.76mol) of 2,4-difluorobenzoyl chloride, 500ml of acetone, and 690g of ammonia water (10.56mol) into a 2000ml single-necked bottle, react at 50°C for 3 hours, concentrate under reduced pressure to remove acetone, filter with suction, and filter cake After drying under reduced pressure, 2,4-difluorobenzamide was obtained, and 255 g (1.62 mol) was obtained, with a yield of 92%.
[0046] Into a 2000ml three-necked flask, add 236g (1.50mol) of 2,4-difluorobenzamide, 500g of tetrahydrofuran, dropwise add 378g (1.80mol...
Embodiment 2
[0047] Embodiment 2: Preparation of 2,4-difluorobenzonitrile
[0048] Add 360g (2.29mol) of 2,4-difluorobenzoic acid and 1091g (9.17mol) of thionyl chloride to a 2000ml single-necked bottle, heat up and reflux for about 5 hours (the temperature of temperature rise and reflux is 78°C), and concentrate to dryness under reduced pressure , to obtain a yellow oil, that is, 2,4-difluorobenzoyl chloride, to obtain 368g (2.08mol), yield 91%.
[0049] Add 353g (2.00mol) of 2,4-difluorobenzoyl chloride, 600ml ethanol, and 785g ammonia water (12.00mol) to a 2000ml single-necked bottle, react at 50°C for 4 hours, concentrate under reduced pressure to remove ethanol, filter with suction, and filter cake After drying under reduced pressure, 2,4-difluorobenzamide was obtained, and 289 g (1.84 mol) was obtained, with a yield of 92%.
[0050]Into a 3000ml three-neck flask, add 283g (1.80mol) of 2,4-difluorobenzamide, 600g of tetrahydrofuran, dropwise add 454g (2.16mol) of trifluoroacetic anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com