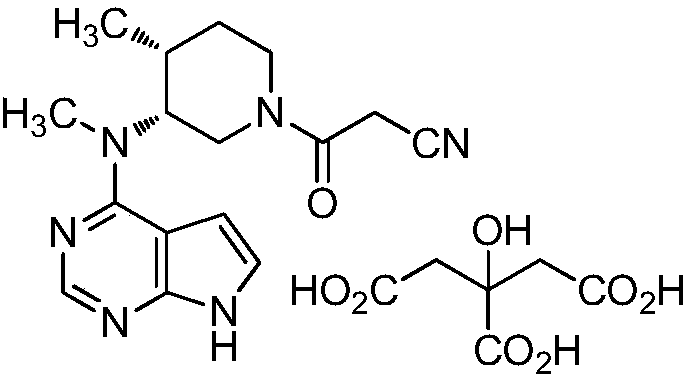
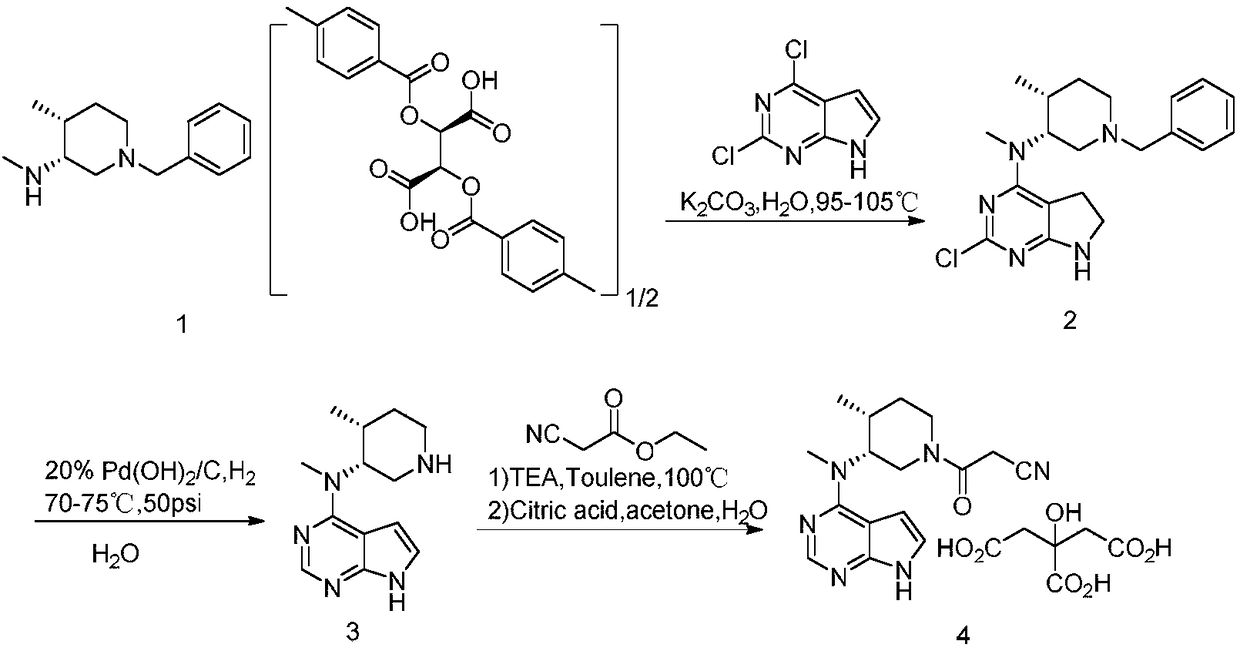
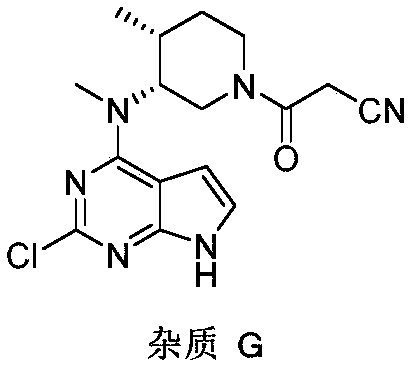
A tofacitinib citrate purification method
A technology of tofacitinib and its refining method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problem of ineffective removal of impurities, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of Tofacitinib Citrate Crude Product
[0026]
[0027] Add 40Kg of purified water into a 100L glass reactor, start stirring, and add 4Kg of (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride (compound VI ), stirring to dissolve the system; then slowly add 9.49Kg of potassium carbonate powder, and stir for 10-20 minutes after the addition; then add 2.61Kg of 2,4-dichloro-7H pyrrole[2,3-D]pyrimidine (compound V). The temperature of the system is controlled at 100±5°C for 20-24 hours. Sampling by HPLC to monitor the reaction. After the reaction is completed, stop heating, cool the system to 20-30°C, centrifuge until almost no solvent comes out, rinse the filter cake with purified water, and centrifuge until no solvent comes out; beat the filter cake with 20Kg purified water for 0.5 hours , Centrifuge until almost no solvent comes out, rinse the filter cake with 8.0Kg purified water, centrifuge until no solvent comes out; add the ...
Embodiment 2
[0032] Embodiment 2 Refining of tofacitinib citrate
[0033] Add 100g of crude tofacitinib citrate (crude product content: 97.64%, impurity G content: 0.44%) and 200 mL of dimethyl sulfoxide into the reaction flask, heat up to 40°C, stir and dissolve until dissolved. Keep 40°C and add 3L of dichloromethane dropwise, after the dropwise addition, slowly cool down to 15°C, a white solid precipitates, keep stirring for 1 hour, filter and dry to obtain 95.0g pure product, yield 95.0%, HPLC detection, impurity G content 0.03% , Tofacitinib citrate purity 99.93%.
Embodiment 3
[0034] Example 3 Refining of Tofacitinib Citrate
[0035] Add 100g of crude tofacitinib citrate (crude content: 97.64%, impurity G content: 0.44%) and 100 mL of dimethyl sulfoxide into the reaction flask, raise the temperature to 45°C, and stir to dissolve until clear. Keep 45°C and add 3L of dichloromethane dropwise, after the dropwise addition, slowly cool down to 10°C, a white solid precipitates, keep stirring for 1 hour, filter and dry to obtain 94.8g of pure product, the yield is 94.8%, the content of impurity G is 0.03% by HPLC detection , Tofacitinib citrate purity 99.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com