Preparation method of leucine dipeptide
A technology of leucine dipeptide and leucine, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of high safety hazards in reaction operations, heavy metal residues, and inability to crystallize and purify, and avoid the Column chromatography, less three wastes, easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
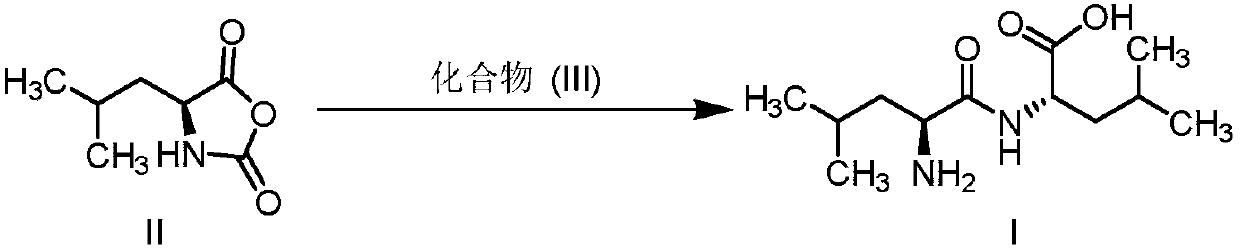
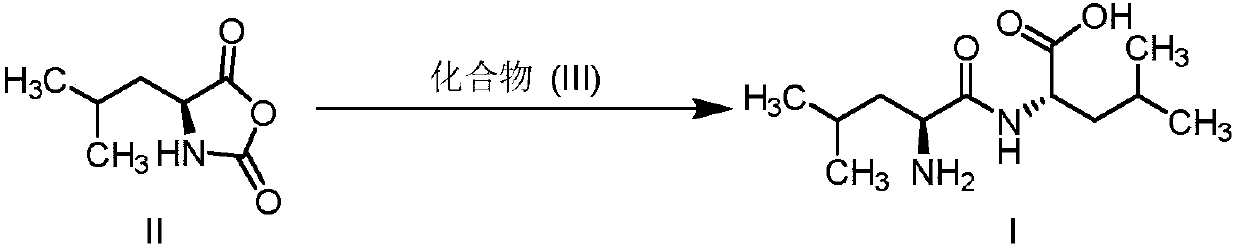
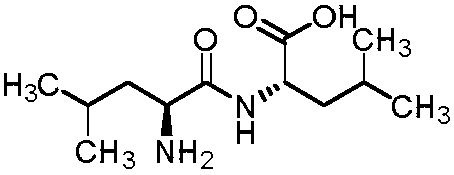
[0025] The preparation of embodiment 1 leucine dipeptide
[0026] Under nitrogen protection, dissolve L-leucine (10g, 76mmol) in acetonitrile, cool to 0-10°C, add potassium carbonate (10.5g, 76mmol), stir for half an hour; dropwise add L-leucine-N- Acetonitrile solution of acid anhydride (11.9g, 76mmol) in the carboxyl ring, after the addition, keep stirring at the temperature for 2h, TLC detects that the reaction is complete, adjust the pH to 5-6 with acetic acid, filter, beat with water, and dry to obtain an off-white solid 16.7g, molar yield 90%, liquid phase purity 98%. 1H NMR (water-d2, 400MHz): δ4.43 (dd, J = 8.8, 5.6Hz, 1H), 4.03 (t, J = 7.0Hz, 1H), 1.86-1.61 (m, 6H), 1.04-0.86 (m,12H).
Embodiment 2
[0027] The preparation of embodiment 2 leucine dipeptide
[0028] Under the protection of nitrogen, dissolve L-leucine (10g, 76mmol) in tetrahydrofuran, cool to 0-10°C, add potassium hydroxide (4.3g, 76mmol), stir for half an hour; dropwise add L-leucine-N - A solution of carboxycyclic anhydride (11.9 g, 76 mmol) in tetrahydrofuran. After the addition, heat up to 20-30°C and stir for 1 hour. TLC detects that the reaction is complete. Adjust the pH to 5-6 with phosphoric acid. After filtering, beat with water. After filtering, dry to obtain 15.8 g of off-white solid with a molar yield of 85%. 98% purity.
Embodiment 3
[0029] The preparation of embodiment 3 leucine dipeptide
[0030] Under nitrogen protection, suspend L-leucine (10g, 76mmol) in toluene, cool to 0-10°C, add potassium carbonate (21g, 152mmol), stir for half an hour; dropwise add L-leucine-N-carboxyl Toluene solution of internal acid anhydride (7.7g, 49.4mmol), after adding, keep stirring at this temperature for 2h, TLC detects that the reaction is complete, adjust the pH to 5-6 with acetic acid, filter and beat with water, filter and dry to obtain off-white solid 10.8g, molar yield 90%, liquid phase purity 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com