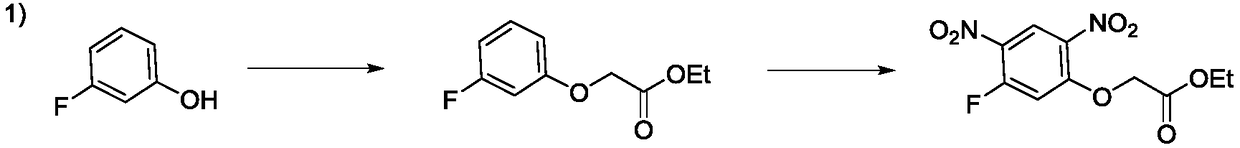
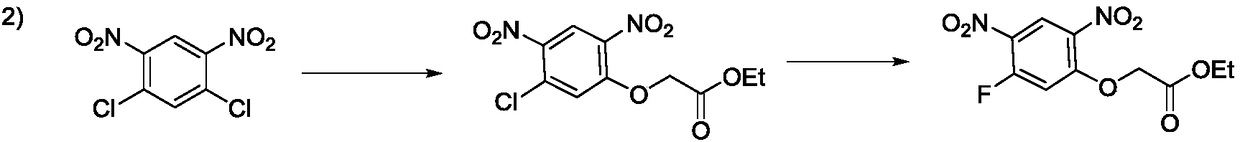
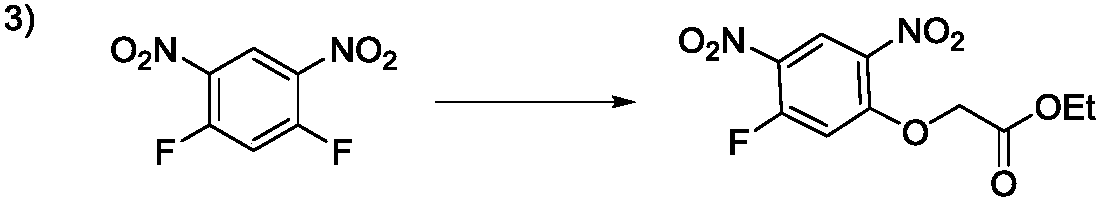
Preparation method of 2-(5-fluoro-2,4-dinitrophenoxy)acetate
A technology of dinitrophenoxy and dinitrophenol, which is applied in the field of preparation of 2-acetate, can solve problems such as poor safety, complicated process, and difficult industrialization, and achieve low production cost, simple reaction, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step 1, add 99g of water into a 250mL reaction kettle at room temperature, slowly add 9.7g (0.17mol) of KOH under magnetic stirring, cool down in an ice-water bath, and then add 1,5-difluoro-2,4-dinitrobenzene (15g , 73.5mmol) was dissolved in 25mL of tetrahydrofuran, and the solution was placed in a constant pressure dropping funnel and slowly added dropwise to the reaction kettle, and a clear light yellow system was obtained after dropping. In the post-processing step, 18g (0.17mol) of 36% HCl was still added dropwise under an ice-water bath. After the dropwise addition, THF was evaporated under reduced pressure, and a large amount of beige solid was precipitated after cooling down. After filtration and drying, 13.5g of solid was obtained, with a yield of 91% and a purity of 99%. %.
[0037] Step 2, 5-fluoro-2,4-dinitrophenol (50.5g, 0.25mol), ethyl chloroacetate (30.5g, 0.25mol), 1,1,1-methyl trichloroacetate (53.2g , 0.3mol), potassium carbonate (0.69g, 5mmol), a m...
Embodiment 2
[0039] Step 1, add 75g of water into a 250mL reaction kettle at room temperature, slowly add KOH12.3g (0.22mol) under magnetic stirring, cool down in an ice-water bath, and then add 1,5-difluoro-2,4-dinitrobenzene (15g , 73.5mmol) was dissolved in 25mL of tetrahydrofuran, and the solution was placed in a constant pressure dropping funnel and slowly added dropwise to the reaction kettle, and a clear light yellow system was obtained after dropping. In the post-treatment process, 22 g of 36% HCl was still added dropwise in an ice-water bath. After the dropwise addition, THF was evaporated under reduced pressure, and a large amount of beige solid was precipitated after cooling down. After filtration and drying, 13.6 g of solid was obtained, with a yield of 92% and a purity of 99%.
[0040] Step 2, 5-fluoro-2,4-dinitrophenol (50.5g, 0.25mol), ethyl chloroacetate (33.5g, 0.28mol), ethyl 1,1,1-trichloroacetate (57.4g , 0.3mol), potassium carbonate (1.38g, 10mmol), a mixture of 18-cro...
Embodiment 3
[0042] Same as in Example 1, using NaOH instead of KOH in step 1 to prepare 2-(5-fluoro-2,4-dinitrophenoxy)acetate, the yield rate is 90% and the purity is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com