Electrochemical preparation method of chloroiridic acid
An electrochemical, chloroiridic acid technology, applied in the direction of electrolysis process, electrolysis components, etc., can solve the problems of increasing preparation steps, many method steps, complex process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
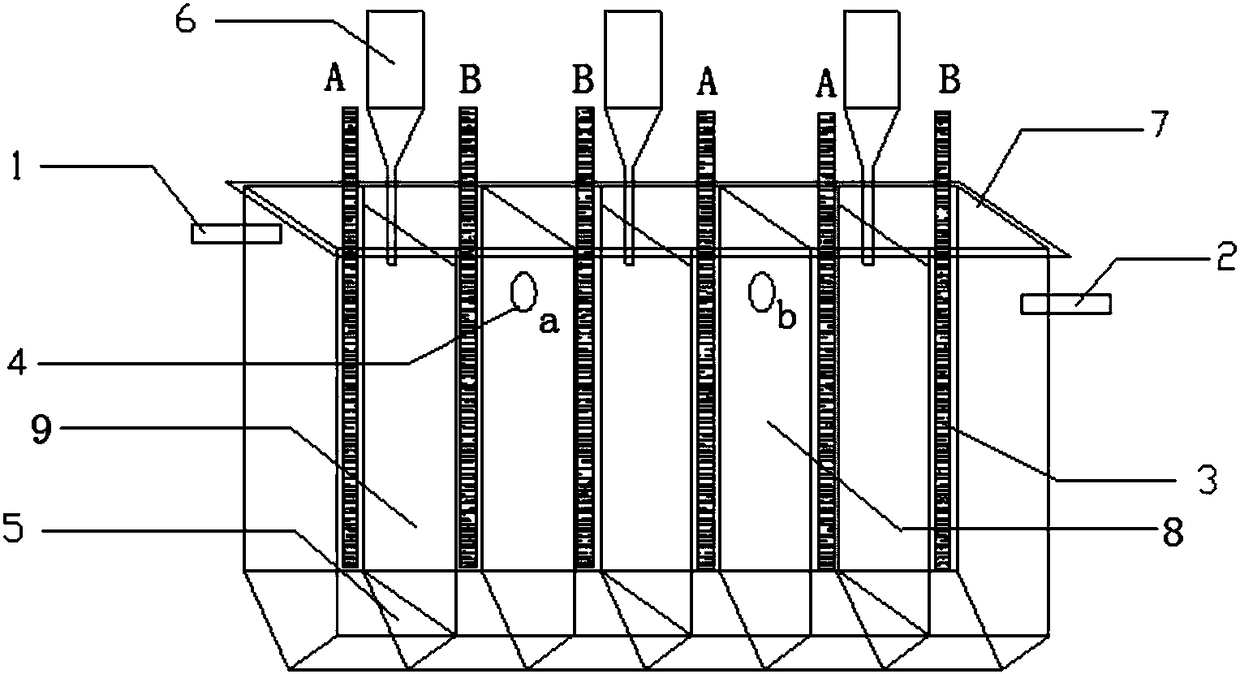
[0043] according to figure 1 The shown structure is provided with an electrolytic cell, and the electrolytic cell is made of quartz glass, and is divided into five tanks by the first dividing plate 8 ( figure 1 Only three tanks are shown in the figure, those skilled in the art can understand that according to figure 1 Five tanks are set in the manner shown). The electrode chamber of the cell body of the electrolytic cell is 55mm long, 40mm wide and 300mm high. The first partition 8 and the second partition 9 are arranged in parallel, the second partition 9 has a gap of 20 mm from the top, and the bottom has a communicating channel 5 with a triangular cross-section. Each cell is divided into two electrode chambers by the second separator 9 . The overflow holes 4 (a, b) are opened on the top of the first partition 8, at a position 50 mm away from the top, and the hole diameter is 10 mm. A hydrochloric acid feed port 1 is arranged on the side wall of the first tank body, a...
Embodiment 2
[0050] Prepare the chloroiridic acid solid in the same manner as in Example 1, the difference is that the electrode chamber of the electrolytic cell body is 60mm long, wide 35mm, and high 320mm; the cross section of the communication channel 5 is a semicircle; during the electrolytic reaction, Apply 30 volts of civilian alternating current at both ends of the A and B electrodes; when the iridium content in the hydrochloric acid was 21 grams per liter, continue to add hydrochloric acid and iridium powder, and control the adding speed of hydrochloric acid to be 11.5 milliliters / minute, according to the consumption of iridium powder Situation adds iridium powder, and the product stream that dissolves 21 gram / liter concentration iridium this moment is discharged from product outlet 2; Finally obtain 58 gram chloroiridic acid solids.
Embodiment 3
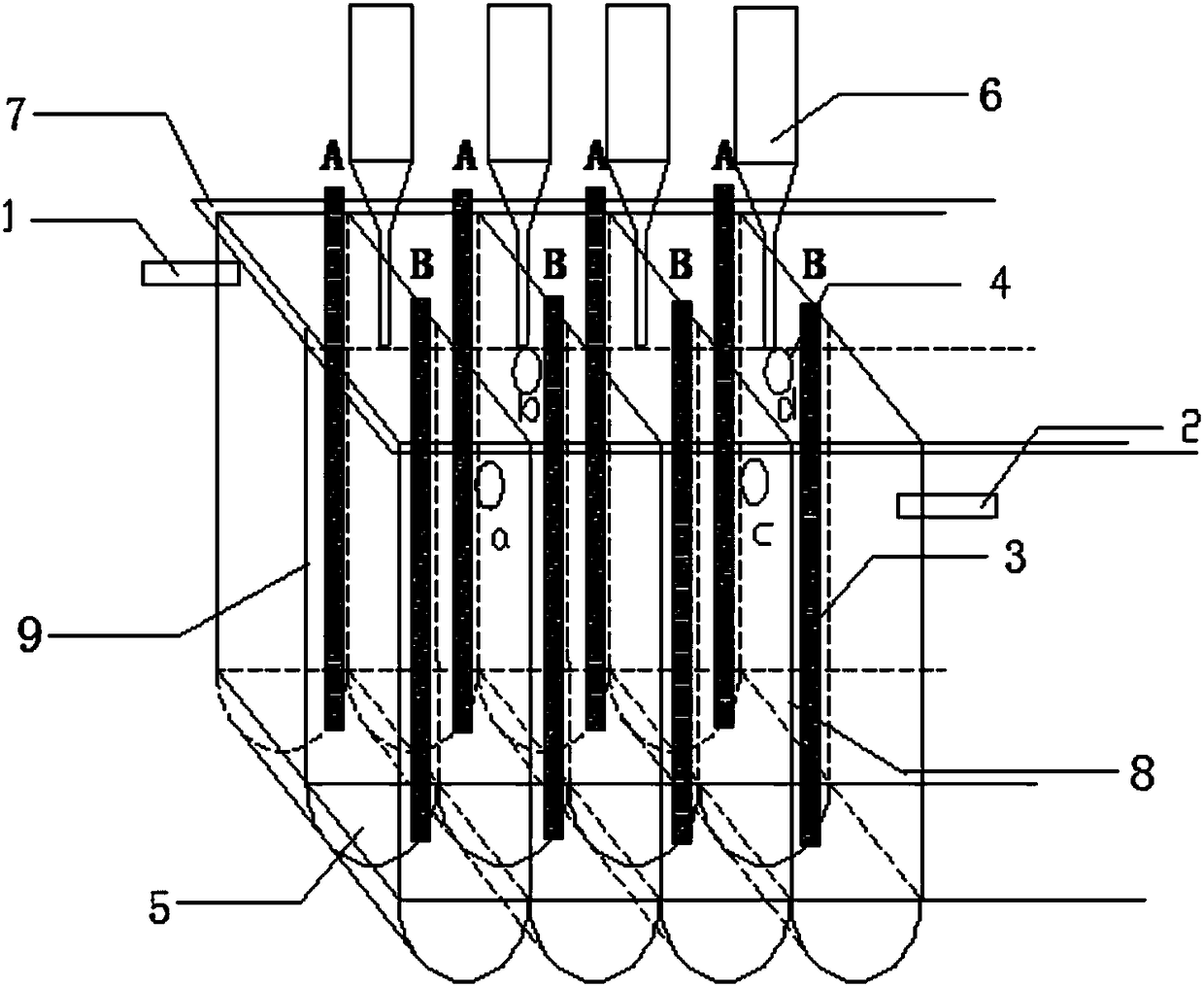
[0052] according to figure 2 The shown structure is provided with an electrolytic cell, and the electrolytic cell is made of quartz glass, and is divided into five tanks by the first dividing plate 8 ( figure 2 Only four tanks are shown in the figure, those skilled in the art can understand that according to figure 2 Five tanks are set in the manner shown). The electrode chamber of the cell body of the electrolytic cell is 58mm long, 40mm wide and 300mm high. The first partition 8 and the second partition 9 are vertically arranged, the second partition 9 has a gap of 20mm from the top, and the bottom has a semicircular communication channel 5 in section. Each cell is divided into two electrode chambers by the second separator 9 . The overflow hole 4 (a, b, c, d) is opened on the upper side of the first partition 8, 50 mm from the top, and the diameter is 10 mm. The overflow hole 4 adjacent to the first partition 8 is opened on the second partition 9 on both sides. A hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com