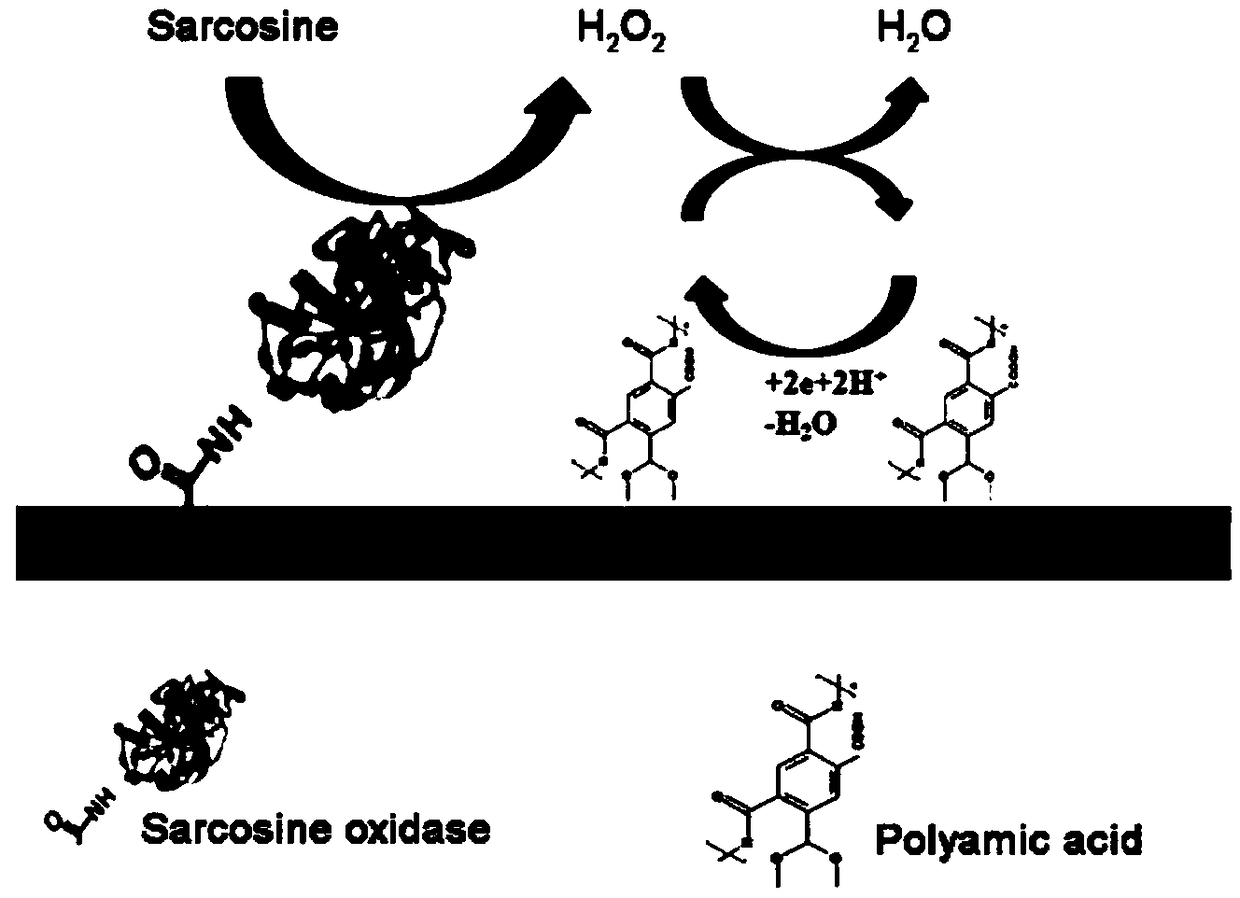
Electrochemical detection method for sarcosine based on polyamide acid and sarcosine oxidase
A technology of sarcosine oxidase and polyamic acid, which is applied in the field of biomedical detection, can solve problems such as the inability to realize sarcosine detection, and achieve significant clinical application value, excellent analytical performance, and great clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Immobilization on polyamic acid (PAA) and sarcosine oxidase (SOX) glassy carbon electrode (GCE)
[0038] The glassy carbon electrode (GCE, diameter 3mm) was pretreated, first using methylformamide (DMF) solution as a solvent to prepare a polyamic acid (PAA) solution with a concentration of 4 mg / mL, and the prepared polyamic acid ( The PAA) solution was sonicated for 30 min to ensure complete dissolution of the PAA, and then 5 μL of the sonicated polyamic acid (PAA) solution was deposited on the GCE surface and dried overnight at room temperature. Subsequently, 5 μL of a uniformly dispersed 2.5% glutaraldehyde solution was dropped onto the surface of the modified GCE and dried at 25 °C for 2 h to form a film. Finally, 5 μL of sarcosine oxidase (SOX) solution (1 mg / mL) was dropped on the modified GCE surface and left overnight at 4 °C to form a SOX layer.
Embodiment 2
[0039] Embodiment 2 electrochemical detection
[0040]Chronoamperometry was carried out in 5mL 0.5M PBS (pH7.2) containing different concentrations of sarcosine, the initial potential was -0.4V, and the sample drop interval was 150s;
[0041] Wherein, the amino acid concentration is (a) 1, (b) 2.5, (c) 5, (d) 10, (e) 25, (f) 50, (g) 75, (h) 100 and (i) 125nM;
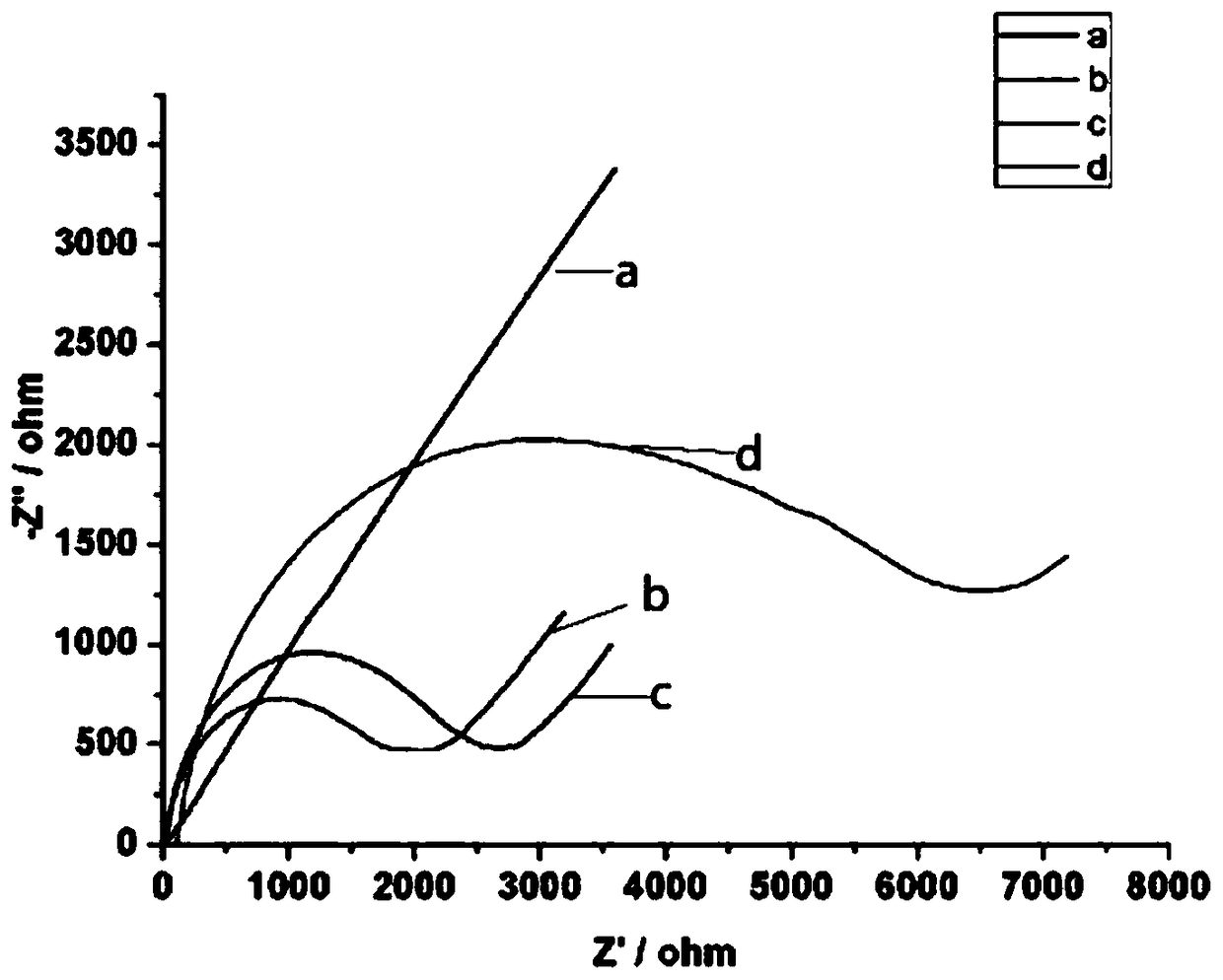
[0042] Electrochemical Impedance Spectroscopy (EIS for short) was used in 5.0mM [Fe(CN) containing 0.1M KCl 6 ] 3- / 4- In solution, the frequency is 10-10 4 Hz.
experiment example 1
[0043] Modification process of experiment example 1 electrode
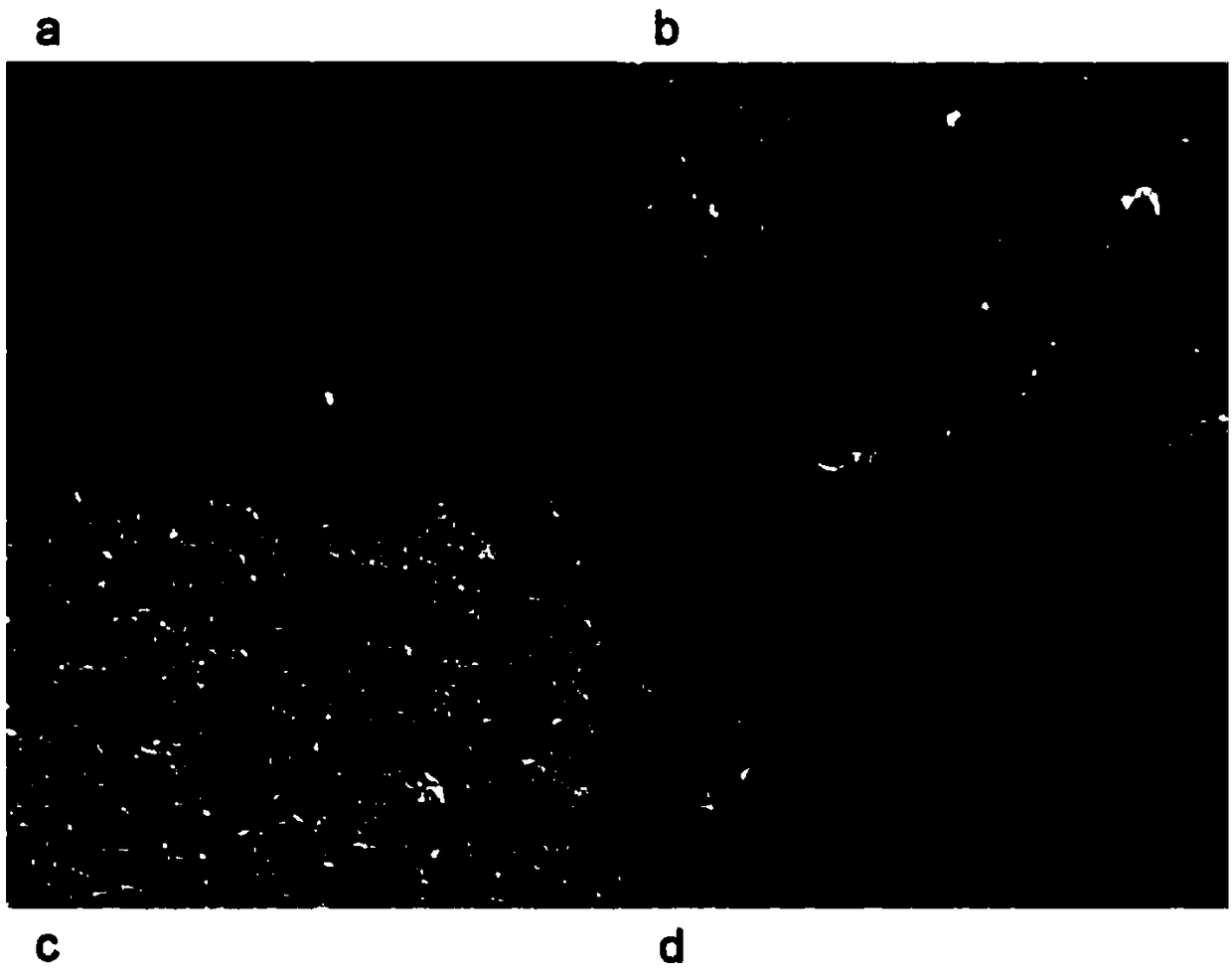
[0044] In this experimental example, the surface morphology of the modified electrode was studied by scanning electron microscopy (SEM). attached figure 2 a shows the topography of the bare GCE; figure 2 b shows PAA / GCE with a uniform PAA layer added dropwise, and the results show that the polymer has covered the electrode surface with good adhesion; figure 2 c shows the SEM image of glutaraldehyde / PAA / GCE with visible thick layer; figure 2 d shows the SEM image of SOX / glutaraldehyde / PAA / GCE, which clearly shows that SOX has been immobilized on the modified electrode surface.
[0045] In order to further verify the modification process of the electrode, this experimental example uses electrochemical impedance spectroscopy (EIS) to verify the modification process of the electrode. as attached image 3 As shown, the EIS of the bare electrode (curve a) is similar to a straight line; after PAA is immobilized,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com