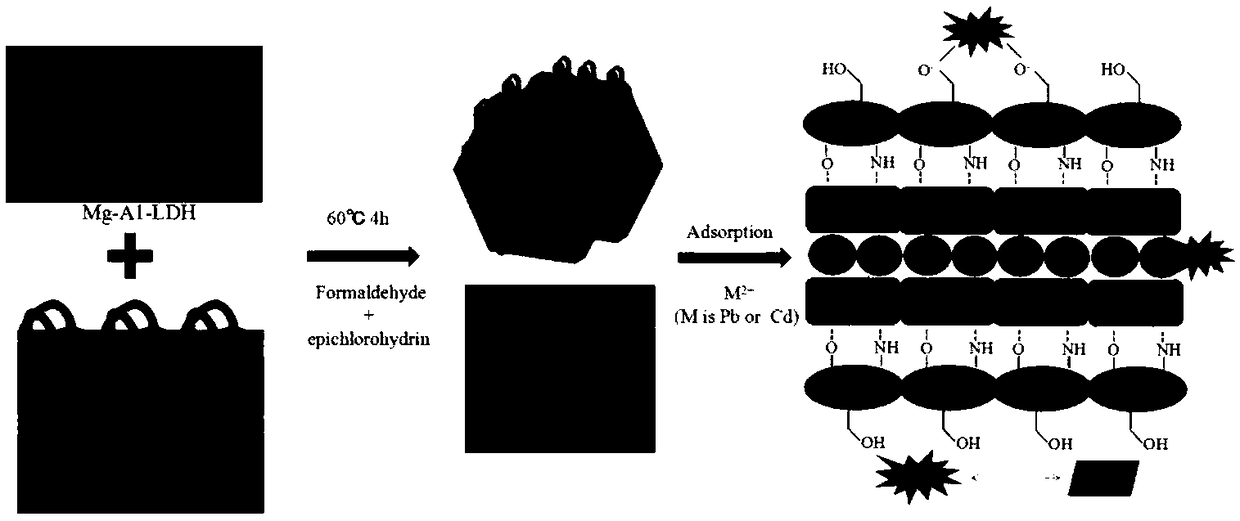
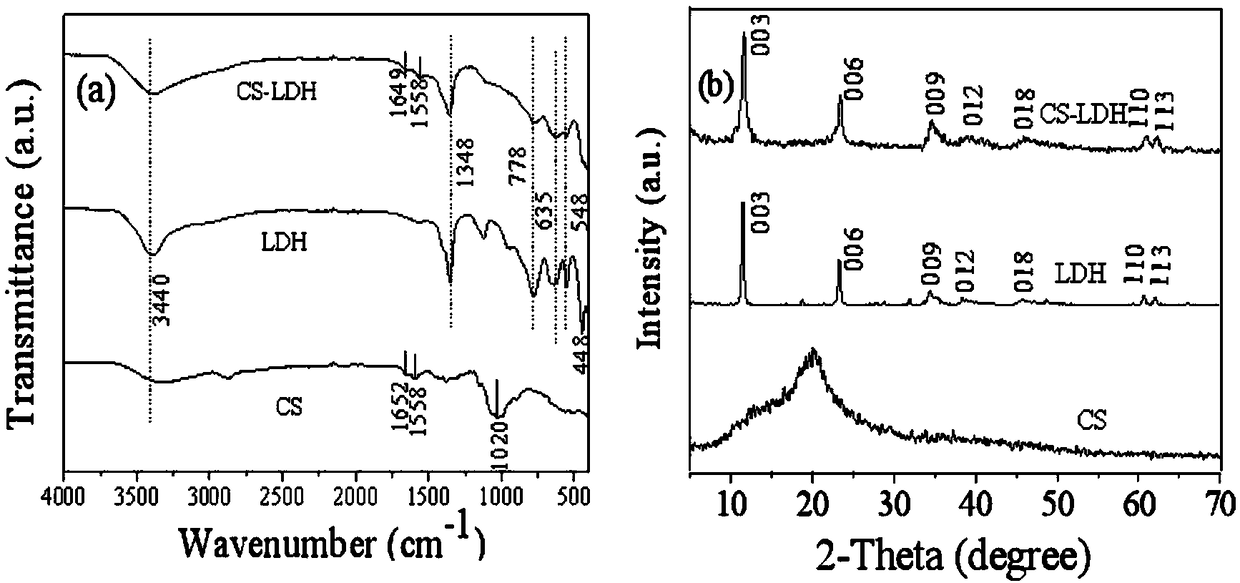
Preparation method of chitosan-hydrotalcite nanocomposite material
A nanocomposite material, chitosan technology, applied in chemical instruments and methods, water pollutants, adsorbed water/sewage treatment, etc., can solve problems such as separation difficulties, influence of adsorption capacity, unfavorable chitosan applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Add 0.5 g of chitosan into 70 ml of 2% acetic acid solution, and dissolve it by ultrasonic to obtain a slightly yellowish transparent solution. Add 0.5-2g magnesium aluminum hydrotalcite into 50ml 2% acetic acid solution to dissolve.
[0041] (2) Put two parts of the solution into a three-hole flask at the same time, stir and react for 1 h. Add 5ml of formaldehyde, raise the temperature to 60°C, and react for 1h. Use 1mol / L NaOH solution to adjust the pH to about 9.0, add 10ml of epichlorohydrin, adjust the pH at any time to keep it at about 9.0, and react for 2.5 hours.
[0042] (3) Wash with absolute ethanol until neutral, and finally dry at 60° C., grind and sieve to obtain white solid chitosan / magnesium aluminum hydrotalcite (CS-LDH).
[0043] At room temperature, add 0.05g CS-LDH to 20ml 100mg / L cadmium nitrate solution, shake for 240min, and measure the adsorption rate. 2+ The removal rate of chitosan to Cd was 72.99%. 2+ The removal rate is 61.01%.
[00...
Embodiment 2
[0054] At room temperature, add a certain mass of CS-LDH to 20ml of 300mg / L Pb 2+ solution, shake at room temperature for 240min, then centrifuge, take the supernatant to detect Pb 2+ Concentration, according to the added Pb 2+ The initial concentration and Pb in the solution after adsorption 2+ The remaining concentration of Pb is calculated 2+ removal rate. Under the same conditions, using chitosan and magnesium aluminum hydrotalcite to adsorb Pb 2+ Conduct a comparative test.
[0055] At room temperature, add a certain mass of CS-LDH to 20ml of 100mg / L Cd 2+ solution, shake at room temperature for 240min, then centrifuge, take the supernatant to detect Cd 2+ Concentration, depending on the added Cd 2+ The initial concentration of and the Cd of the solution after adsorption 2+ The remaining concentration of the calculated Cd 2+ removal rate. Under the same conditions, chitosan and magnesium aluminum hydrotalcite were used to adsorb Cd 2+ Conduct a comparative test...
Embodiment 3
[0065] Add 0.05g CS-LDH to 20ml 300mg / L Pb at room temperature 2+ In the solution, adjust different pHs, shake for 240 minutes, and calculate the removal rate and adsorption capacity (see Table 4 for details).
[0066] Add 0.05g CS-LDH to 20ml 100mg / L Cd at room temperature 2+ In the solution, adjust different pHs, shake for 240 minutes, and calculate the removal rate and adsorption capacity (see Table 5 for details).
[0067] Table 4 pH on CS-LDH adsorption of Pb 2+ Impact
[0068]
[0069]
[0070] Table 5 pH on CS-LDH adsorption of Cd 2+ Impact
[0071]
[0072] From Table 4, 5 and Figure 9 It can be seen that the adsorption of heavy metal ions by CS-LDH is less affected by pH.
[0073] 4. Adsorption of heavy metal ions of different concentrations by CS-LDH
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com