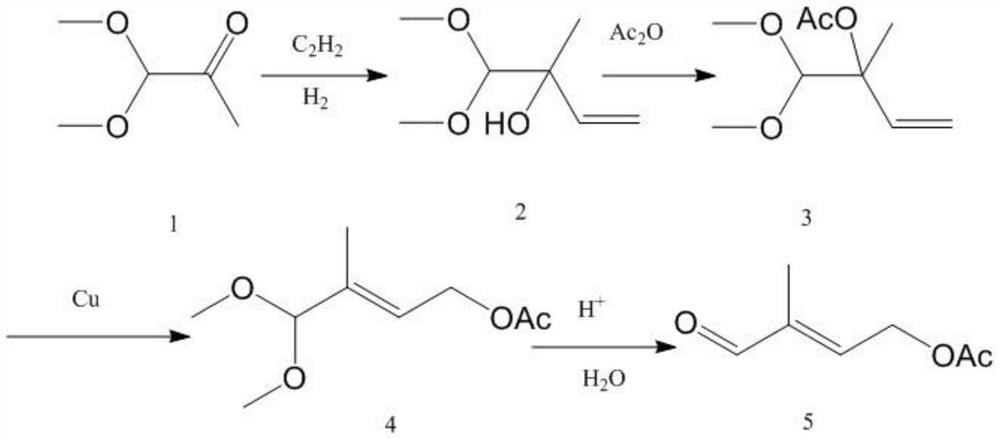
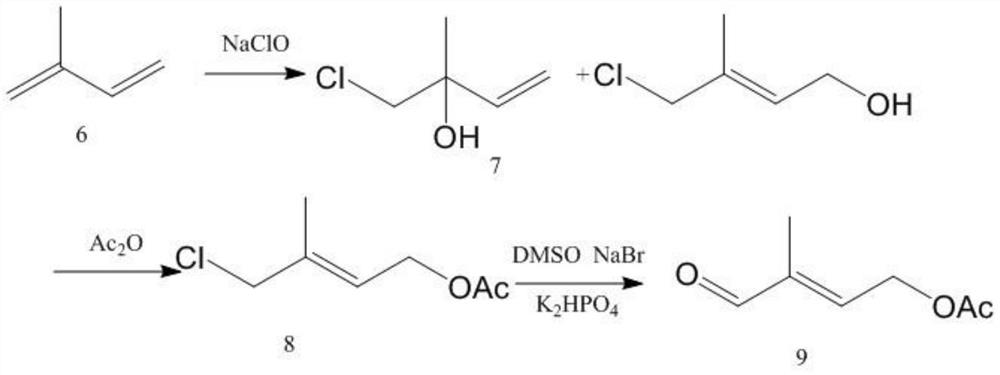
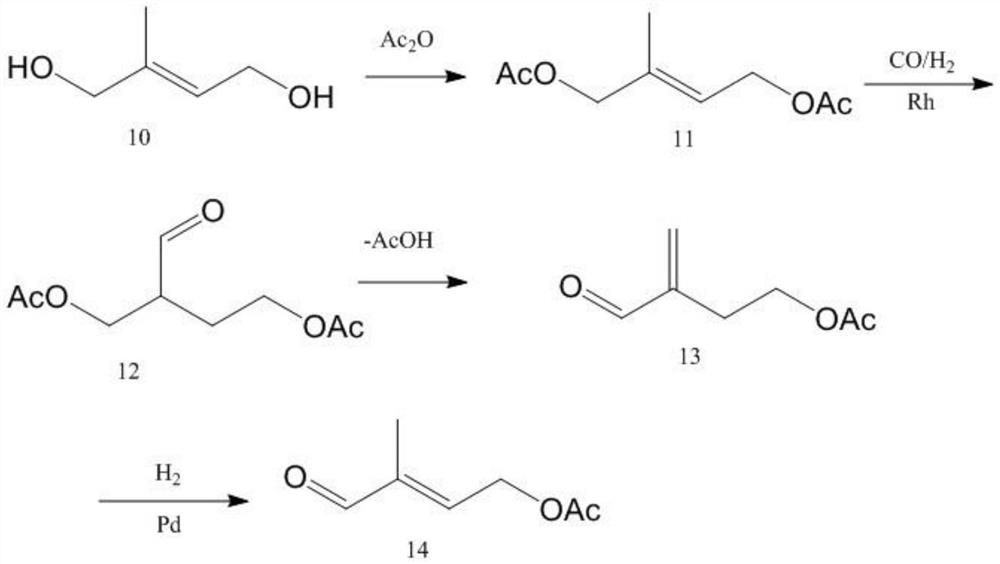
A kind of preparation method of 4-acetoxy-2-methyl-2-butenal
A technology of acetoxy and crotonaldehyde, applied in the field of preparation of 4-acetoxy-2-methyl-2-butenal, which can solve the problems of consumption price, a large number of by-products, unstable intermediates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Preparation of prenol acetate: Add 20 mol of deionized water, 1.1 mol of anhydrous sodium acetate, and 0.02 mol of benzyltriethylammonium iodide into a 2L glass three-necked flask, and stir with a glass rod until the solid is dissolved in water After all the solution is dissolved, the temperature is raised to 45°C, 1 mol of chloroisoamylene is added to the flask, the high-speed shear stirring motor is turned on, and the rotation speed is set at 5000 rpm to start the reaction. After reacting for 4 hours, the liquid in the flask was cooled and transferred to a 2L separatory funnel. After standing for 2 hours, the liquid was separated, and the upper organic phase was taken for gas chromatography analysis. The conversion rate of isopentyl chloride was 95.56%. The enol acetate selectivity was 98.55%. Use a packed column with a theoretical plate number of 25 to rectify the organic phase and remove the solvent from the reaction solution under the condition of 1KPa. The ref...
Embodiment 2
[0068] (1) Preparation of prenol acetate: Add 20 mol of deionized water, 1.1 mol of anhydrous sodium acetate, and 0.02 mol of trioctylmethylammonium iodide in a 2L glass three-necked flask, and stir with a glass rod until the solid is dissolved in water After all dissolved, raise the temperature to 35°C, add 1mol chloroisoamylene into the flask, turn on the high-speed shear stirring motor, set the rotation speed to 5000rpm, and start the reaction. After reacting for 4 hours, the liquid in the flask was cooled and transferred to a 2L separatory funnel. After standing for 2 hours, the liquid was separated, and the upper organic phase was taken for gas chromatography analysis. The conversion rate of isopentyl chloride was 96.15%. The selectivity of enol acetate was 97.93%. Use a packed column with a theoretical plate number of 25 to rectify the organic phase and remove the solvent from the reaction solution under the condition of 1KPa. The reflux ratio is 1:1, and the fraction at...
Embodiment 3
[0071] (1) Preparation of prenol acetate: Add 40 mol of deionized water, 1.2 mol of anhydrous lithium acetate, and 0.05 mol of tetrabutylammonium iodide into a 2L glass three-necked flask, and stir with a glass rod until the solid is completely dissolved in water Afterwards, the temperature was raised to 40° C., 1 mol of chloroisoamylene was added into the flask, the high-speed shear stirring motor was turned on, and the rotation speed was set to 5000 rpm to start the reaction. After reacting for 4.5 hours, the liquid in the flask was cooled and transferred to a 2L separatory funnel. After standing for 2 hours, the liquid was separated, and the upper organic phase was taken for gas chromatography analysis, and the conversion rate of isoamyl chloride was 97.82%. The selectivity of pentenol acetate is 95.86%. Use a packed column with a theoretical plate number of 10 to rectify the organic phase and remove the solvent from the reaction solution under the condition of 0.8KPa. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com